| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 11, Number 5, October 2020, pages 294-304
Model-Informed Development of Sotalol Loading and Dose Escalation Employing an Intravenous Infusion
John C. Somberga, c, Alexander A. Vinksb, Min Dongb, Janos Molnara
aAmerican Institute of Therapeutics, 21 N Skokie Hwy, Suite G-3, Lake Bluff, IL 60044, USA
bDivision of Clinical Pharmacology, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Avenue, MLC6018, Cincinnati, OH 45229, USA
cCorresponding Author: John C. Somberg, American Institute of Therapeutics, 21 N Skokie Hwy, Suite G-3, Lake Bluff, IL 60044, USA
Manuscript submitted July 27, 2020, accepted August 3, 2020, published online August 7, 2020
Short title: IV and Oral Loading of Sotalol
doi: https://doi.org/10.14740/cr1143
| Abstract | ▴Top |
Background: Sotalol is often employed to prevent recurrence of symptomatic atrial flutter/atrial fibrillation. Because sotalol can prolong the QT interval excessively causing ventricular arrhythmias, a 3-day in-hospital loading or dose escalation period is mandated with oral administration in the product label for patient safety. In patients with normal renal function, 3 days (five oral doses) are required to obtain steady state maximum sotalol concentration, which results in maximum QT prolongation. The aim of this study is to develop an intravenous to oral loading regime for sotalol therapy that reduces the 3-day in-hospital initiation or dose escalation with oral administration to 1 day without compromising patient safety.
Methods: Using model-informed drug development techniques, simulations were developed for initiation and dose escalation of sotalol therapy by employing an intravenous loading dose followed by oral sotalol administrations.
Results: In patients with normal renal function, an initial 1-h loading dose of intravenous sotalol followed by two oral doses in 24 h has been developed permitting attainment of three maximum serum concentrations reflecting maximum QT prolongation in a 1-day observation period. Dosing regimens for patients with impaired renal function are also developed.
Conclusions: In patients with normal renal function, using an intravenous loading dose followed by oral administrations permits safe initiation or dose escalation of sotalol in 1 day instead of the 3-day dosing regimen with oral administration.
Keywords: Sotalol; Intravenous sotalol; Simulations; Dose initiation; Dose escalation
| Introduction | ▴Top |
Sotalol is a frequently employed therapy to prevent the recurrence of very symptomatic atrial fibrillation (AF), or atrial flutter (AFL) [1, 2]. Because sotalol can prolong the QTc interval excessively [3, 4] and thus may lead to the initiation of torsades de pointes (TdP) ventricular tachycardia (VT) [3-5], initiation of sotalol therapy has been recommended in the product label to be in-hospital under electrocardiogram (ECG) monitoring with facilities and personnel able to provide cardiac resuscitation [4]. Given the half-life of sotalol being on average 10 - 12 h, 3 hospital days are needed to reach a steady state (Cmax) blood concentration of sotalol. The blood concentration of sotalol directly relates to the prolongation in the QTc interval and thus proarrhythmia risk [3, 4]. The peak blood concentration reached at the steady state (Cmax ss) is the highest concentration and thus offers greatest risk. Three hospital days in a telemetry bed is a considerable expenditure of time and resources for the loading of sotalol. The economic cost is considerable and has been estimated at $9,263 (CMS reimbursement for 3-day initiation of sotalol) [6]. In addition to cost, there is the allocation of resources and the increased exposure of patient to nosocomial acquisition of infections. We hypothesize that intravenous (IV) loading of sotalol followed by oral dosing in quick successions could achieve a sotalol blood concentration similar to that achieved with chronic oral dosing. In this way, a patient under ECG observation could be evaluated as to the QTc response to peak blood sotalol level, and from that the QTc effect could be determined and thus the extent of risk of sotalol therapy would be anticipated. The IV sotalol load followed by the first and second oral dose could be administered in 24 h with risk assessed under ECG monitoring in appropriate hospital facilities. This would shorten hospitalization, reduce patient inconvenience, reduce cost and reduce unneeded hospital exposure of the patient.
| Materials and Methods | ▴Top |
This is a modeling study using previously published data. The published data had the Institutional Review Board (IRB) approval, which is not needed for this study.
We initially approached the problem of 1-day IV and oral loading of sotalol with a proposed study to the Food and Drug Administration (FDA). In consultation with the Cardio Renal Division of FDA, development was proposed along the lines of initiating a model-informed drug development (MIDD) pathway.
A previously performed bioequivalence study in 15 healthy volunteers aged 18 to 45 years old who received a single dose of oral and IV sotalol (crossover study) was employed to obtain serum sotalol concentration and corresponding QTc measurements [7]. A software package NONMEM™ version 7.2 (ICON, Hanover, MD, USA) was used for population pharmacokinetic (PK) modeling and simulation. First order conditional estimation method with interaction (FOCE-INTER) was used for computation. The R Foundation for Statistical Compiling was used for data preparation, graphical analysis, linear regression analysis and statistical analysis and summaries.
A detailed publication of the modeling methodologies are reported elsewhere [8]. A joint population pharmacokinetic/pharmacodynamic (PK-PD) model with covariance between PK and PD parameters was employed. Models were evaluated for best fit by goodness-of-fit diagnostic plots, condition number and decrease in objective fraction value. The performance of the population PK-PD models was further evaluated using “boot strap” analysis and predictive check. The development population PK-PD model was utilized for simulations to determine IV loading doses of sotalol that matched with targeted Cmax ss levels associated with the oral (PO) dosing regimen of 80, 120 and 160 mg PO twice a day (bid). These principles were applied to develop dose loading and dose escalation parameters, as well as modification of dose loading and escalation for patients with different degrees of renal function.
While innumerable possibilities exist as to the IV loading of sotalol followed by oral drug administration, we chose to administer the IV load over 1 h to Cmax ss target, to facilitate physicians following QTc changes from baseline every 15 min, as well as evaluating changes in heart rate (HR) and blood pressure (BP) over a convenient time period. We next hypothesized that an oral sotalol dose should follow the infusion at such a time as to bring the sotalol blood level back to Cmax ss. The first oral dose would then be followed by a second oral dose at a pre-determined interval once again bringing the sotalol blood level back to Cmax ss. We planned that in 24 h or less, three Cmax ss concentrations of sotalol levels would be obtained to evaluate the drug’s effect on QT interval. We expected that some patients would show excess QTc prolongation (500 ms or greater, or a 20% increment in the QTc from baseline) such that dosing would need to be reduced, or sotalol therapy abandoned for alternative therapy.
| Results | ▴Top |
Modeling the parameters for 1-h IV infusion to target Cmax ss we obtained an infusion dose of sotalol based on the oral dosing schedule (Table 1). For 80 mg PO bid oral dosing, one would administer 60 mg to achieve an average Cmax ss target of 800 ng/mL, followed by 5 h from start of infusion of 80 mg taken orally and then 12 h after the first oral dose another 80 mg (17 h from start of infusion). Each oral dose will peak in 2 - 4 h following administration, permitting a check of HR, BP and QTc effects. Thus in a 21 h period, one has three sotalol peak concentrations that permits evaluation of QTc response and thus possible proarrhythmia. The simulation for loading to 80 mg PO bid is depicted in Figure 1. Loading to 120 mg sotalol bid dosing in patients with normal renal function is noted in Figure 2.
 Click to view | Table 1. Recommended Loading Dosages for Initiation and Dose Escalation of Sotalol Administration |
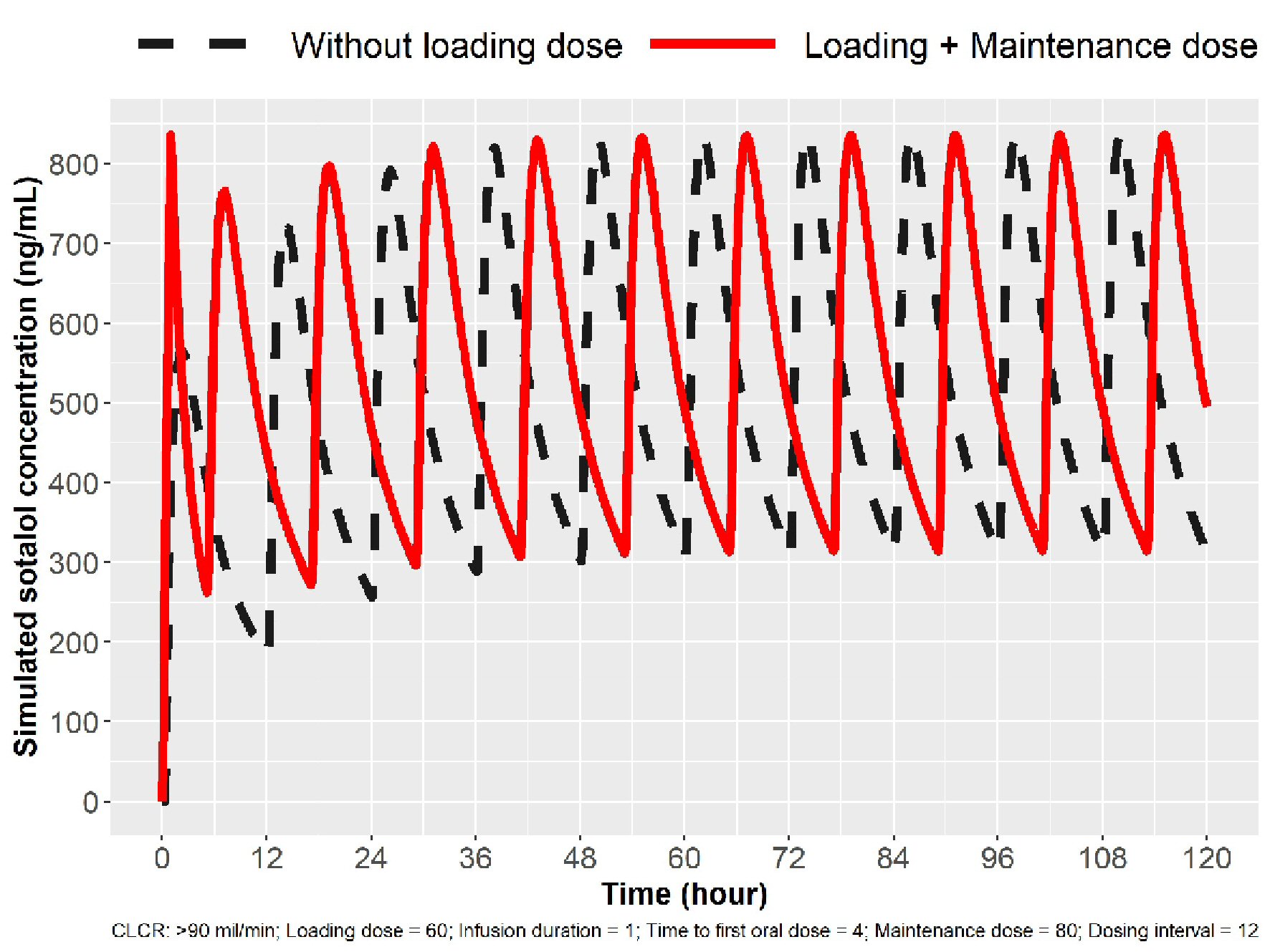 Click for large image | Figure 1. Simulation for 80 mg loading in patients with normal renal function (ClCr > 90 mL/min). The broken line indicates sotalol concentrations with oral (PO) dosing. The solid line indicates sotalol concentrations following IV loading, and Cmax ss concentration can be obtained in 1 h and three Cmax peaks in 24 h. IV: intravenous; ClCr: creatinine clearance. |
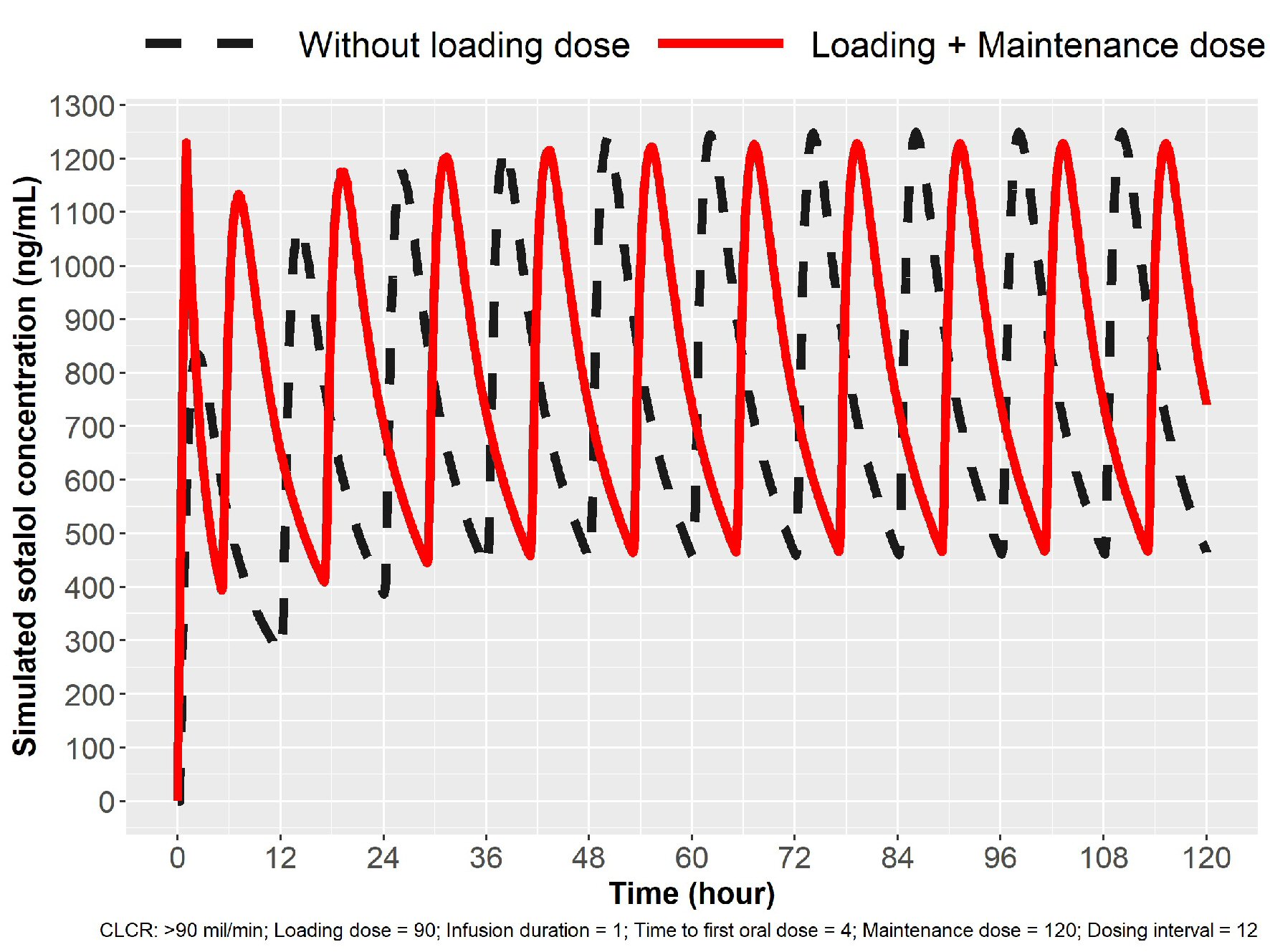 Click for large image | Figure 2. Simulation for 120 mg loading in patients with normal renal function (ClCr > 90 mL/min). Oral dosing 120 mg sotalol every 12 h (broken line) and IV loading 90 mg over 1 h followed by oral dosing. Cmax ss was obtained in 1 h with IV loading. IV: intravenous; ClCr: creatinine clearance. |
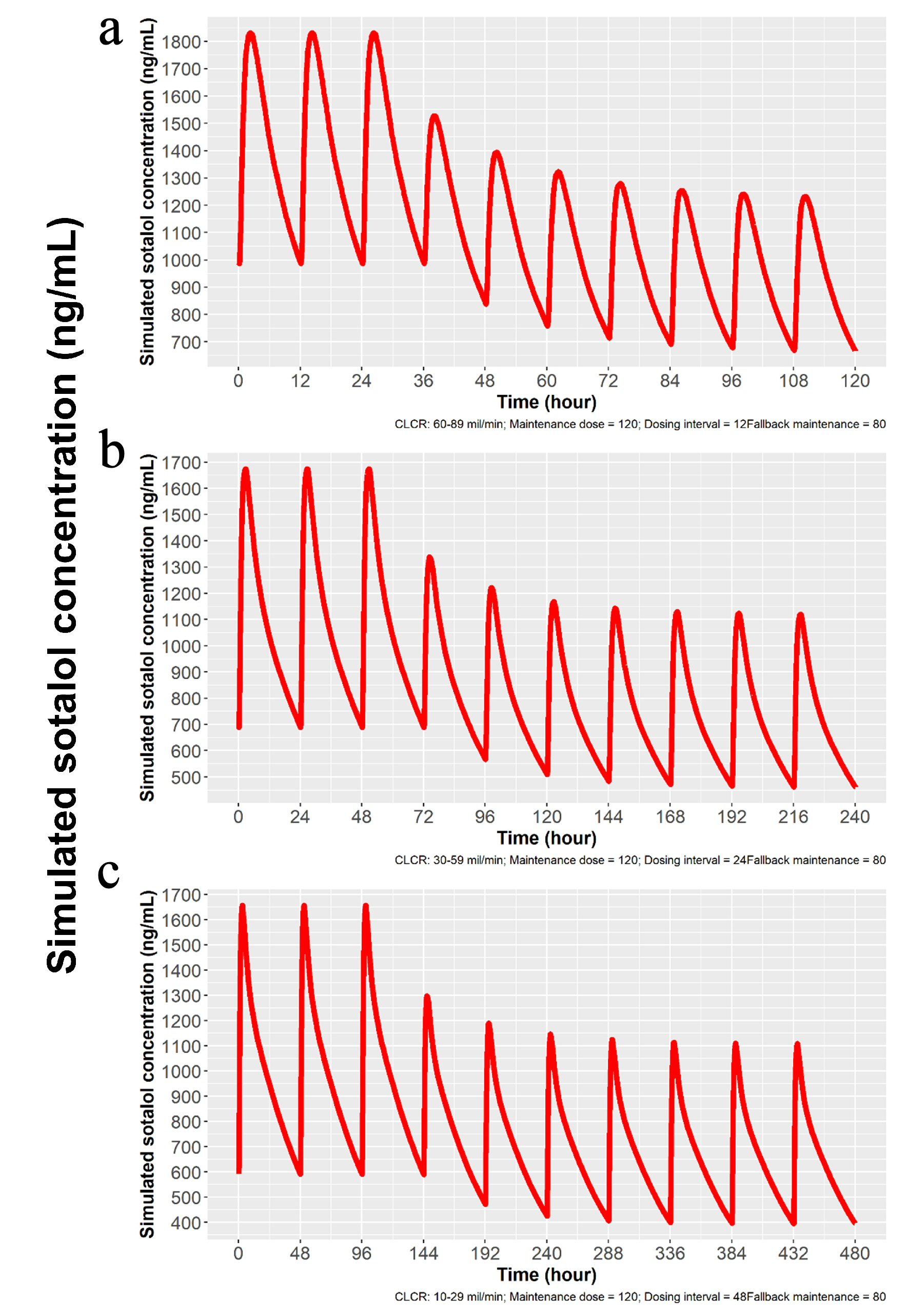
Since sotalol is known to be mostly excreted by the kidney unchanged [9] and elimination is directly proportional to glomerular filtration [10], we developed based on modeling and simulation technique, doses to obtain predicted Cmax for patients with mild, moderate and severe renal dysfunction. Proposed dosing is listed in Table 1 and simulations are depicted in Figures 3 and 4. Sotalol administration in patients with severe renal function was limited in the label to patients with a history of life-threatening VT.
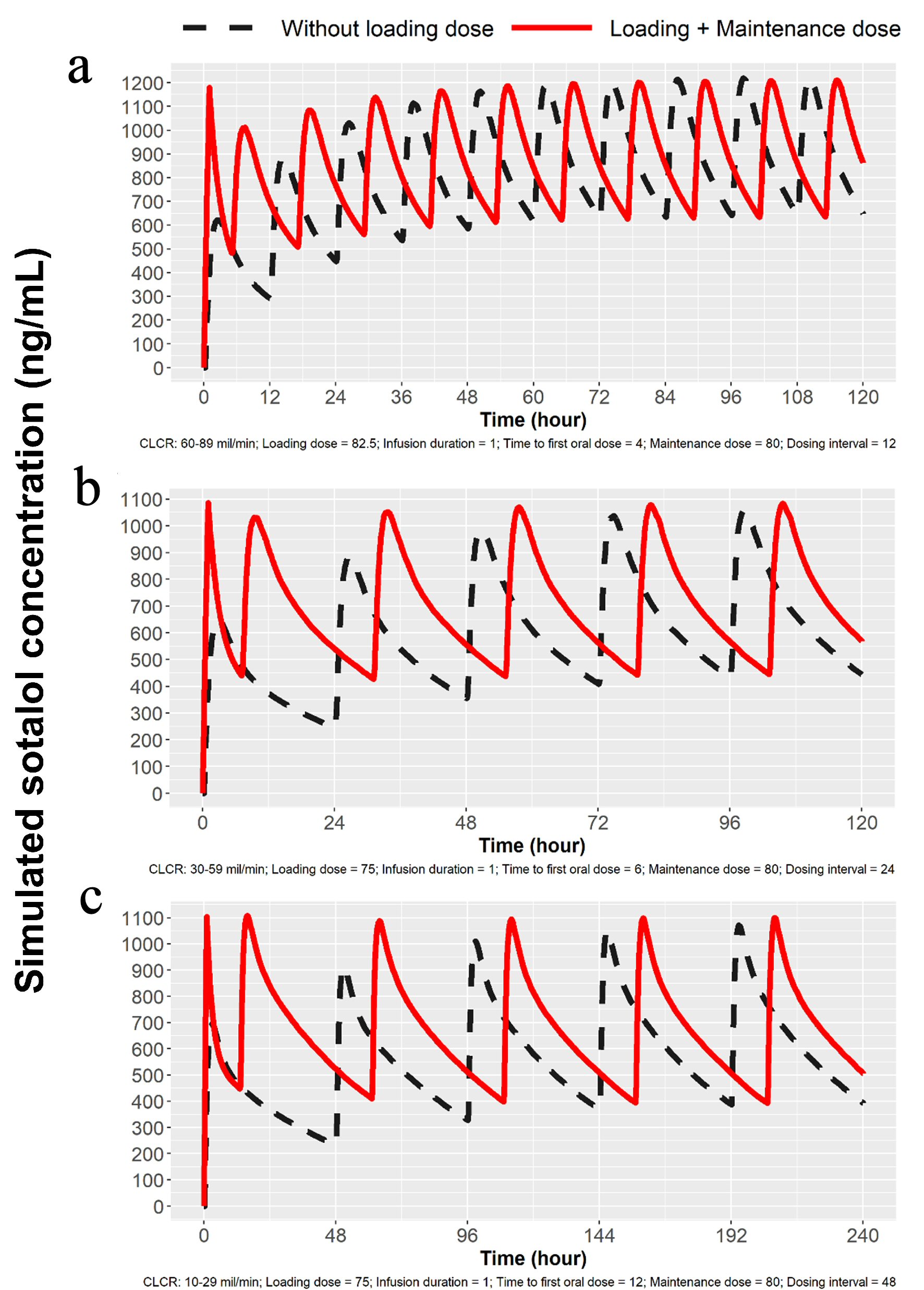 Click for large image | Figure 3. Simulations for 80 mg dosing for patients with mild, moderate and severe renal impairment. (a) Simulation with mild renal impairment (ClCr: 60 - 89 mL/min). Oral loading broken lines and solid line with 82.5 mg IV load. (b) Simulation for moderate renal impairment (ClCr: 30 - 59 mL/min). Broken line indicates oral loading and solid line 75 mg IV load, followed by 80 mg PO at 7 h and then 80 mg PO every 24 h. (c) Simulation for severe renal impairment (ClCr: 10 - 29 mL/min). Broken line indicates oral loading 80 mg PO every 48 h, while solid line represents 75 mg IV followed by 80 mg PO at 13 h and then every 48 h thereafter. IV: intravenous; ClCr: creatinine clearance; PO: oral. |
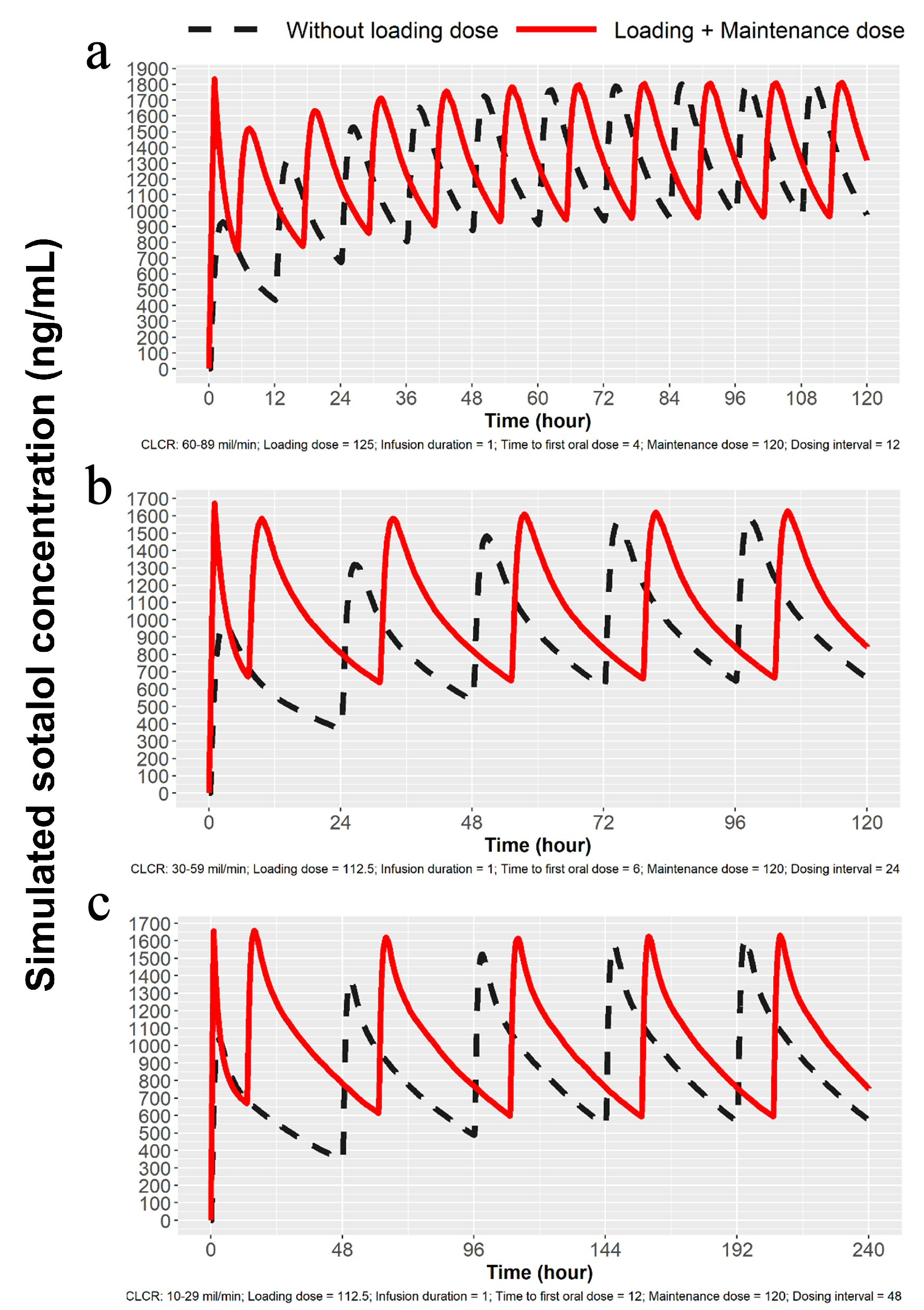 Click for large image | Figure 4. Simulations for 120 mg dosing for patients with mild, moderate and severe renal impairment. (a) Simulation for mild renal impairment (ClCr: 60 - 89 mL/min). Broken line represents 120 mg sotalol PO every 12 h and the solid line represents 125 mg IV load over 1 h followed by 120 mg at 5 h PO and then every 12 h thereafter. (b) Simulation for moderate renal impairment (ClCr: 30 - 59 mL/min). Broken line represents 120 mg sotalol PO administered every 24 h and solid line represents 112.5 mg IV over 1 h followed by 120 mg at 7 h and then 120 mg PO every 24 h. (c) Simulation for severe renal impairment (ClCr: 10 - 29 mL/min). Broken line represents120 mg sotalol orally every 48 h and the solid line 112.5 mg IV over 1 h followed by 120 mg orally at 13 h and then 120 mg PO every 48 h. IV: intravenous; ClCr: creatinine clearance; PO: oral. |
In addition to loading there is the need for dose escalation. Patient may be on oral sotalol 80 mg PO bid or 120 mg PO bid and because of breakthrough atrial fibrillation, escalation to the next higher dose may be desired to accomplish achieving a higher concentration. Simulations were created to escalate from 80 mg to 120 mg bid or 120 mg to 160 mg in 1 day using IV loading. The initial IV load for escalation is listed in Table 1. Simulations for 1-day dose escalation are presented in Figures 5 and 6 for the two dose escalations, for patients with normal renal function. Patient with impaired renal function can also undergo dose escalation with doses given for initial IV loading and then times to first and second oral doses (Table 1). Simulations are presented for dose escalation in patients with mild, moderate and severe renal function (Fig. 7).
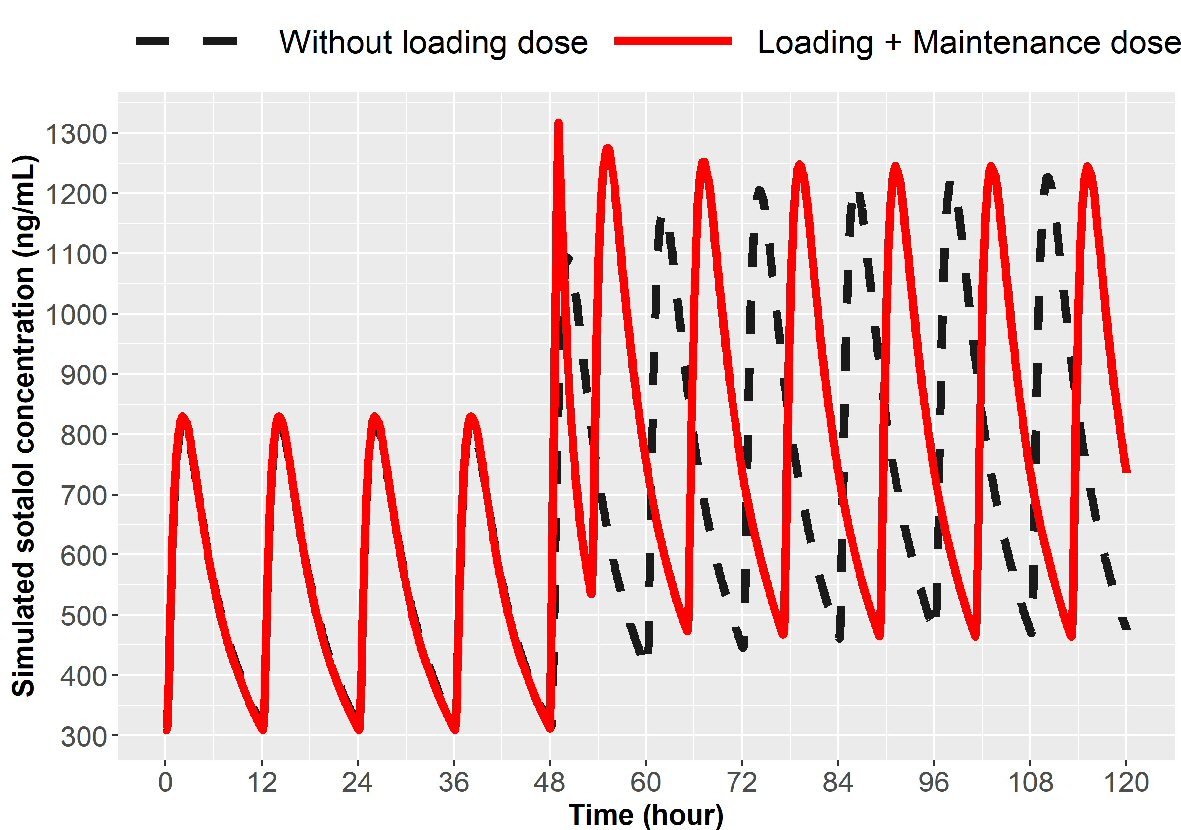 Click for large image | Figure 5. Simulation for dose escalation from 80 to 120 mg in patients with normal renal function (ClCr > 90 mL/min). Broken line represents oral loading, 120 mg every 12 h and the solid line represents 75 mg IV over 1 h given at 12 h after the last 80 mg PO dose followed by 120 mg oral at 5 h and then every 12 h thereafter. IV: intravenous; ClCr: creatinine clearance; PO: oral. |
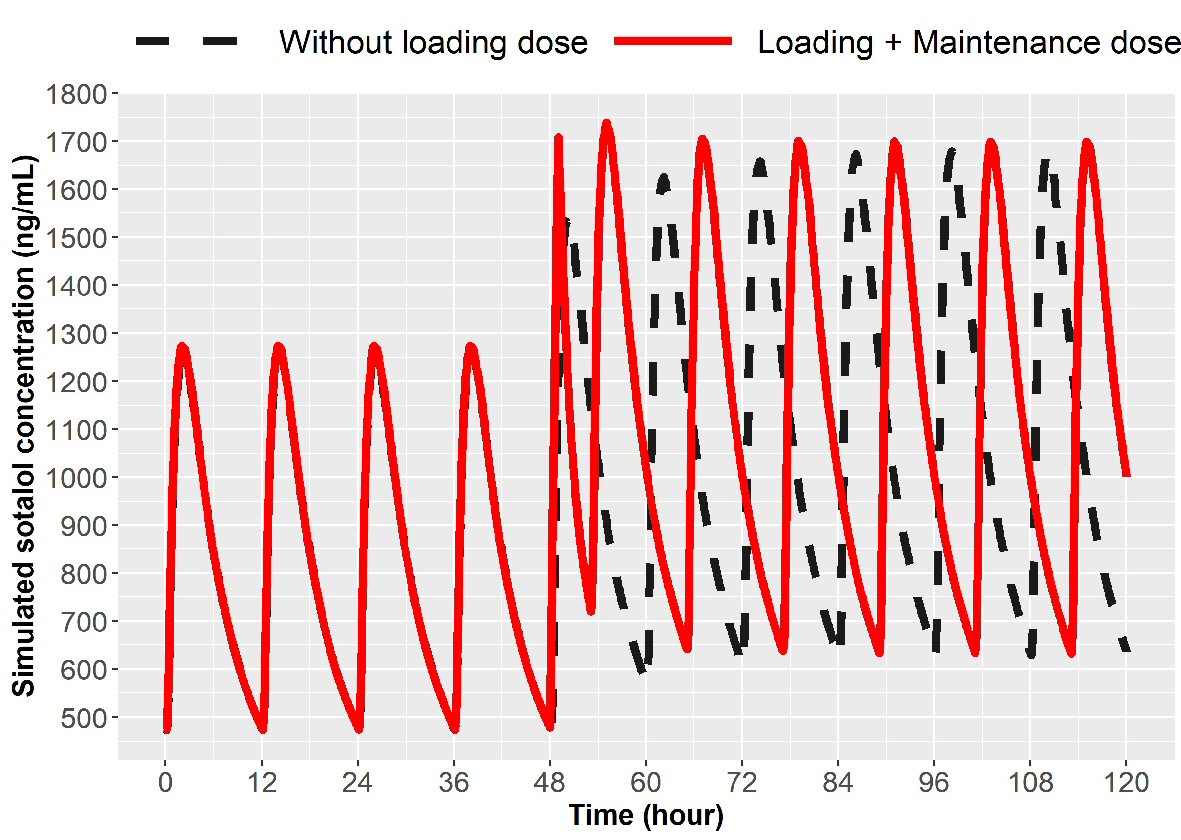 Click for large image | Figure 6. Simulation for dose escalation from 120 to 160 mg in patients with normal renal function (ClCr > 90 mL/min). The broken line represents 160 mg PO sotalol 12 h after the last 120 mg PO dose and then 160 mg at every 12 h thereafter. The solid line represents 90 mg IV sotalol loading over 1 h given 12 h after the last 120 mg dose followed by 160 mg PO at 5 h and then every 12 h thereafter. IV: intravenous; ClCr: creatinine clearance; PO: oral. |
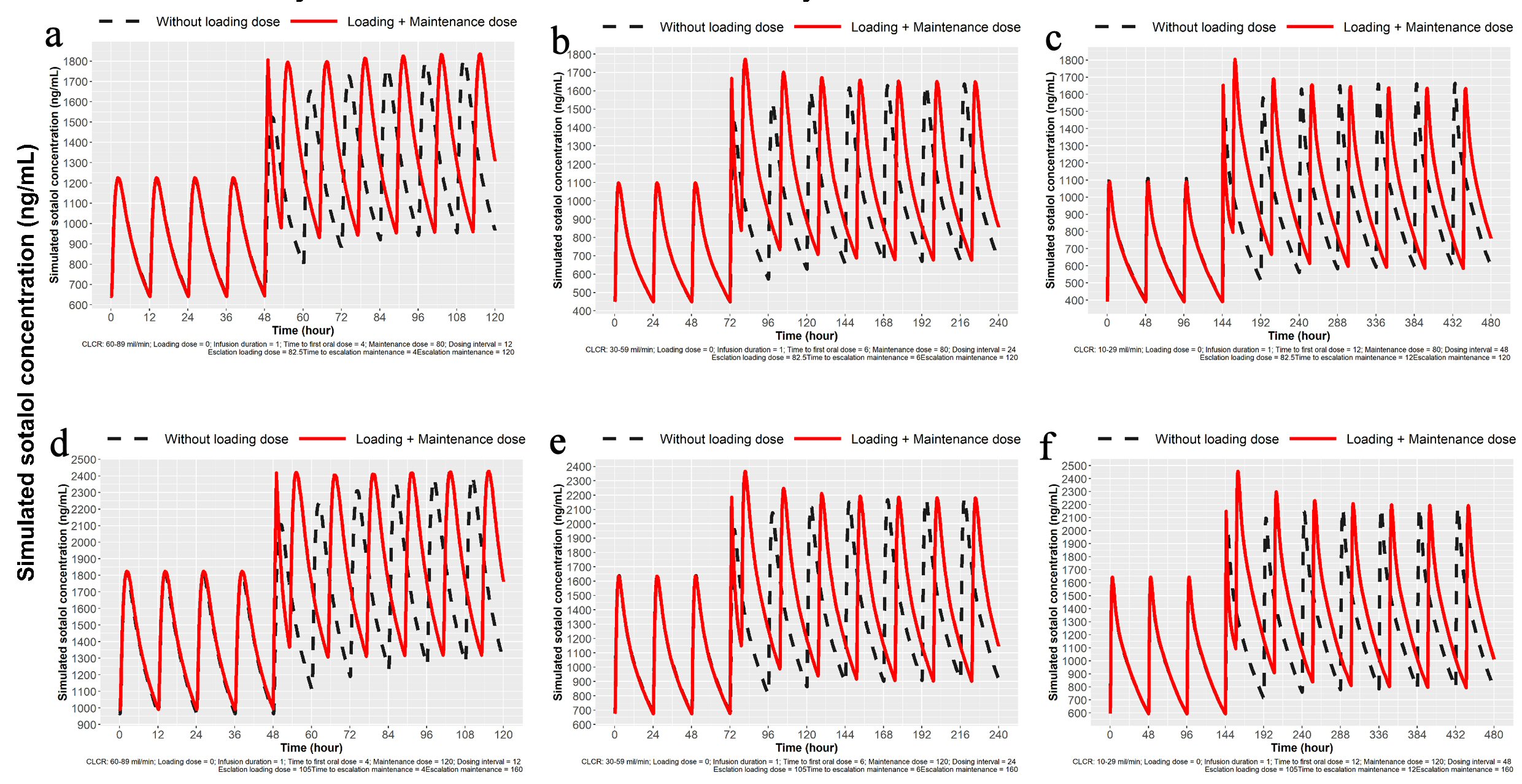 Click for large image | Figure 7. Simulation for dose escalation from 80 to 120 mg and from 120 to 160 mg sotalol for patients with mild, moderate and severe impairment of renal function. (a) Simulation for dose escalation from 80 to 120 mg in patients with mild renal impairment (ClCr: 60 - 89 mL/min). Oral loading 120 mg PO at 12 h after the last 80 mg PO dose and then 120 mg every 12 h. IV loading 82.5 mg IV load over 1 h, 12 h after the last 80 mg PO dose followed by 120 mg at 5 h and then 120 mg PO every 12 h. (b) Simulation for moderate renal impairment (ClCr: 30 - 59 mL/min). Oral loading 120 mg sotalol PO at 24 h after the last 80 mg PO dose and then every 24 h. IV loading 82.5 mg over 1 h at 12 h after the last 80 mg PO dose followed by 120 mg at 7 h and then every 24 h. (c) Simulation for severe renal impairment (ClCr: 10 - 29 mL/min). Oral loading 120 mg sotalol oral 48 h after the last 80 mg PO dose and then 120 mg every 48 h. IV loading of 82.5 mg IV over 1 h at 48 h after the last 80 mg PO dose followed by 120 mg PO at 13 h and then every 48 h. (d) Simulation for dose escalation from 120 to 160 mg in patients with mild renal impairment (ClCr: 60 - 89 mL/min). Oral loading 160 mg sotalol PO at 12 h after the last 120 mg PO dose and then 160 mg at every 12 h. IV loading of 90 mg IV over 1 h at 12 h after the last 120 mg PO dose followed by 160 mg PO at 5 h from the start of the infusion and then 160 mg PO every 12 h. (e) Simulation for moderate renal impairment (ClCr: 30 - 59 mL/min). Oral loading 160 mg sotalol at 24 h after the last 120 mg PO dose and then 160 mg PO every 24 h. IV loading of 105 mg IV load administered over 1 h at 24 h after the last 120 mg PO dose followed by 160 mg PO at 7 h and then 160 mg PO every 24 h. (f) Simulation for severe renal impairment (ClCr: 10 - 29 mL/min). Oral loading 160 mg sotalol at 48 h after the last 120 mg PO dose and then 160 mg every 48 h. IV loading of 105 mg IV over 1 h at 48 h after the last 120 mg PO dose followed by 160 mg PO at 13 h and then 160 mg PO every 48 h. IV: intravenous; ClCr: creatinine clearance; PO: oral. |
A patient could be initiated with a loading dose to 120 mg PO bid (Cmax target), exhibit excessive QTc prolongation and thus be placed on a lower dose of 80 mg twice daily. Using modeling and simulation to guide re-initiation of sotalol at a lower dose of 80 mg PO bid could be obtained by waiting 1 day (in patients with creatinine clearance (ClCr) > 60 mL/min), or at least 3 days in patients with ClCr < 60 or ≥ 30 mL/min, or 7 days in patients with ClCr < 30, but ≥ 15 mL/min. Simulations for dose “fall back” strategy are depicted in Figures 8 and 9.
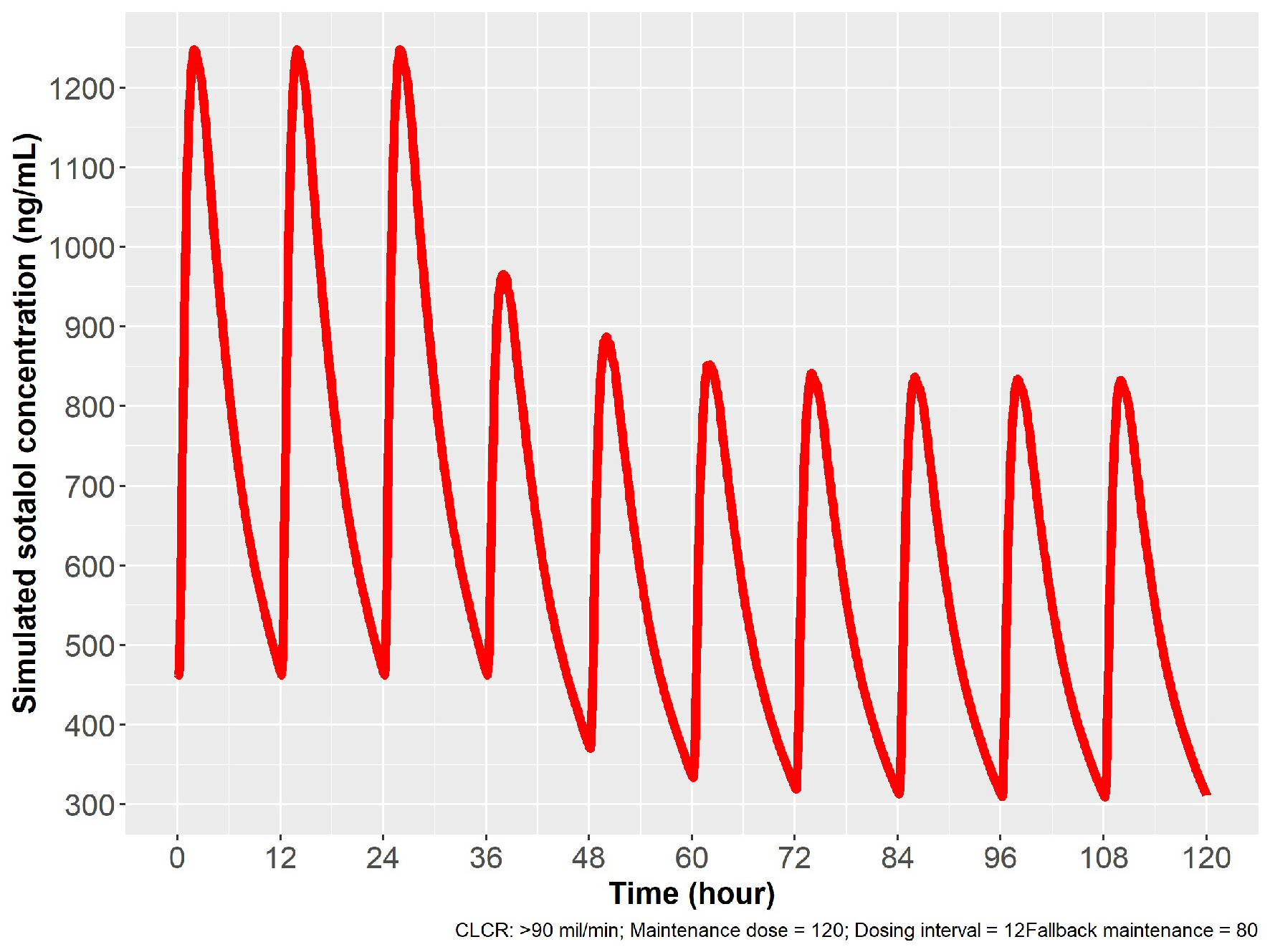 Click for large image | Figure 8. Simulation for dose reduction from 120 to 80 mg PO sotalol in patients with normal renal function (ClCr: 90 mL/min). After 12 h from the last 120 mg dose, 80 mg PO given with a new steady state achieved in 24 h, with 80 mg given every 12 h. ClCr: creatinine clearance; PO: oral. |
 Click for large image | Figure 9. Simulation for dose reduction from 120 to 80 mg PO sotalol for patients with mild, moderate and severe impairment of renal function. (a) Simulation for patients with mild renal impairment (ClCr: 60 - 89 mL/min). After the last 120 mg dose, 80 mg is given in 12 h, and a new steady state is achieved in 48 h, with 80 mg dosing every 24 h thereafter. (b) Simulation for patients with moderate renal impairment (ClCr: 30 - 59 mL/min). Twenty-four hours after the last 120 mg dose, 80 mg is given and new steady state is reached in 72 h with 80 mg dosing every 24 h. (c) Simulation for patients with severe renal impairment (ClCr: 10 - 29 mL/min). Forty-eight hours after stopping the last dose of 120 mg, a dose of 80 mg is given and a new steady state is obtained in 96 h, with 80 mg dosing every 48 h thereafter. ClCr: creatinine clearance; PO: oral; |
| Discussion | ▴Top |
Employing model-informed drug development approaches, simulations were developed to employ IV sotalol infusion over 1 h to initially load patients who are to receive oral sotalol therapy to prevent recurrence of highly symptomatic AF/AFL. Since sotalol can prolong the QTc and excessive prolongation may lead to TdP VT in 3% of patients [11], early determination of the QTc effect at maximum blood concentrations of sotalol (Cmax) is requisite to avoid proarrhythmic risk. Sotalol blood concentration is known to be directly proportional to the QTc prolongation [11, 12]. Testing the Cmax that is seen with chronic oral therapy with a 1-h infusion of IV sotalol permits a rapid evaluation of proarrhythmic risk, as well as HR and BP effects. With the first and second oral doses, a second and third sotalol concentration peak is achieved that further permits evaluation of the QTc effects in-hospital under careful monitoring, permitting appropriate dose reduction or therapy termination. While there is little evidence suggesting a delay in the QTc prolonging effects of sotalol, a hysteresis effect may occur in some patients, which would be observed following the second or third doses. That the effects of the greatest future sotalol blood concentration that will be obtained on oral dosing can be observed three times in patients with normal renal function in a 24 h period, provides assurance as to the safety of the selected oral dosing of sotalol for chronic oral therapy.
One can take the time concentration sotalol curves and transform them directly into a time QTc prolongation curve in QT variance [3, 4, 11, 12]. However, this represents the average response. Certain patients may exhibit an exaggerated response with a given QTc prolongation being considerably longer than the mean response. These patients may be at the greatest risk for proarrhythmia. The exaggerated response may be due to potassium channel abnormalities. However, one must be cautious in that non-excessive QT prolongation can still lead to significant proarrhythmia in patients with hypokalemia, severe bradycardia, and extensive myocardial disease that are associated with a low ejection fraction.
While there was reluctance on the part of regulators to provide for loading to 120 mg initially, having both regimens available to initiate sotalol, offers considerable advantage. Many physicians chose to start with 120 mg PO bid, since this dose has the greatest likelihood of maintaining sinus rhythm [13]. Having this option for initial oral loading will facilitate adoption of the IV sotalol loading strategy.
The model revealed that patients with mild, moderate and severe renal dysfunction have an average higher maximum serum sotalol concentration than patients with normal renal function, despite adjustments in dosing based on ClCr. Because of these higher concentrations, a larger loading dose of sotalol is required in these groups to assess the QTc response to a higher concentration of sotalol with chronic oral therapy. If physicians want to have their patients with reduced renal function receive a reduced maximum sotalol serum concentrations, then a lower maintenance dose of sotalol should be selected. When a lower chronic dose is selected, then a reduced “test” IV dose can be employed. It is the target dosing that dictates the concentration of the initial IV loading.
Conclusions
Using pharmacokinetic modeling, the loading and dose escalation schedules were developed for patients who are to receive chronic oral sotalol. Dosing regimens were adjusted for renal function. Patient in-hospital evaluation could be accomplished in 24 h in patients with normal renal function.
Acknowledgments
Authors thank to Issam Zineh, PharmD, MPH, Norman Stockbridge, MD, PhD, and their associates at the FDA: Karen Hicks, MD, Charu Gandotra, MD, Ramana Uppoor, PhD, Sudharshan Hariharan, PhD, Girish Bende, PhD, Yaning Wang, PhD, Hao Zhu, PhD, Chao Liu, PhD, Luning (Ada) Zhuang, PhD, Rajnikanth Madabushi, PhD, Sharonjit Sagoo, PharmD, CPH, David Strauss, MD, PhD, Renmeet Grewal, PharmD, MS, Jessica Benjamin, MPH, Tracey Lee, BS, RN, MSN, Yvonne Knight, MS, Christopher Egelebo, Million Tegenge, PhD, Jacqueline Glen, MSc, Vivianna Cowl, Ququan Liu MD, MS, for their guidance and model modification.
Financial Disclosure
This work has been sponsored by AltaThera Pharmaceuticals, Chicago, IL, USA.
Conflict of Interest
JCS developed IV sotalol and licensed it to AltaThera Pharmaceuticals. AAV: paid consultant to AltaThera Pharmaceuticals. MD: paid consultant to AltaThera Pharmaceuticals. JCS and JM have a patent that has been assigned to the sponsoring company, AltaThera Pharmaceuticals.
Informed Consent
This is a modeling study working from previously published data. There is no need for informed consent in this paper. The published papers previously mentioned informed consent.
Author Contributions
All authors contributed to the writing of this paper.
Data Availability
The data supporting the findings of this study are available in the published literature cited in this study.
| References | ▴Top |
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr., Conti JB, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-267.
- The Top 300 Drugs of 2020. Available at: https://clincalc.com/DrugStats/Top300Drugs.aspx.
- Soyka LF, Wirtz C, Spangenberg RB. Clinical safety profile of sotalol in patients with arrhythmias. Am J Cardiol. 1990;65(2):74A-81A; discussion 82A-83A.
doi - Sotalol hydrochloride injection. Prescribing Information. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ff3061ab-d930-4318-a5be-684e38be229e.
- MacNeil DJ, Davies RO, Deitchman D. Clinical safety profile of sotalol in the treatment of arrhythmias. Am J Cardiol. 1993;72(4):44A-50A.
doi - Bunch TJ, May H, Bair T, Steinberg BA, Muhlestein JB, Anderson JL, Knowlton K. Economics of sotalol in-patient dosing approaches in patients with atrial fibrillation. JACC. 2020;75(Suppl1):e362.
doi - Somberg JC, Preston RA, Ranade V, Molnar J. Developing a safe intravenous sotalol dosing regimen. Am J Ther. 2010;17(4):365-372.
doi pubmed - Dong M, Fukuda T, Selim S, Smith MA, Rabinovich-Guilatt L, Cassella JV, Vinks AA. Clinical trial simulations and pharmacometric analysis in pediatrics: application to inhaled loxapine in children and adolescents. Clin Pharmacokinet. 2017;56(10):1207-1217.
doi pubmed - Hanyok JJ. Clinical pharmacokinetics of sotalol. Am J Cardiol. 1993;72(4):19A-26A.
doi - Antonaccio MJ, Gomoll A. Pharmacology, pharmacodynamics and pharmacokinetics of sotalol. Am J Cardiol. 1990;65(2):12A-21A; discussion 35A-36A.
doi - Sotalol hydrochloride tablet prescribing information. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=62a71c8c-d92d-4122-848f-9ae35f8bbe2b.
- Somberg JC, Preston RA, Ranade V, Molnar J. QT prolongation and serum sotalol concentration are highly correlated following intravenous and oral sotalol. Cardiology. 2010;116(3):219-225.
doi pubmed - Benditt DG, Williams JH, Jin J, Deering TF, Zucker R, Browne K, Chang-Sing P, et al. Maintenance of sinus rhythm with oral d,l-sotalol therapy in patients with symptomatic atrial fibrillation and/or atrial flutter. d,l-Sotalol Atrial Fibrillation/Flutter Study Group. Am J Cardiol. 1999;84(3):270-277.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


