| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Case Report
Volume 13, Number 4, August 2022, pages 242-245
Ivabradine Overdose in a Newborn: Precautions of Dispensing in Infants
Pezad Doctora, William A. Scotta, Kaitlin Tindelb, Hoang H. Nguyena, c
aDepartment of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA
bDepartment of Pharmacy, Dallas Children’s Medical Center, Dallas, TX 75235, USA
cCorresponding Author: Hoang H. Nguyen, Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA
Manuscript submitted May 7, 2022, accepted May 27, 2022, published online August 15, 2022
Short title: Ivabradine Overdose in a Newborn
doi: https://doi.org/10.14740/cr1392
| Abstract | ▴Top |
Ivabradine is currently approved to reduce heart rate in children with chronic heart failure and dilated cardiomyopathy. Ivabradine has also been used off-label in children to treat automatic tachyarrhythmias such as ectopic atrial tachycardia and junctional ectopic tachycardia. Adverse effects of ivabradine at physiological doses as well as its toxicity at supra-physiological doses have rarely been reported in adults. In children, weight-based dosing requires dilution of commercially available ivabradine oral solution for accuracy. We describe a case of ivabradine overdose in a newborn (treated for ectopic atrial tachycardia) secondary to inaccurate dosing leading to the infant receiving 10 times more drug than prescribed. This case highlights potential pitfalls of ivabradine prescription and preparation in children.
Keywords: Ivabradine toxicity; Ectopic atrial tachycardia; Newborn; Sinus bradycardia
| Introduction | ▴Top |
Ivabradine reduces the heart rate by inhibiting the cardiac pacemaker current responsible for the spontaneous depolarization of the sinoatrial node [1]. Ivabradine is currently indicated in the treatment of heart failure and angina in adults, and in heart failure and dilated cardiomyopathy in children [2, 3]. Recently, ivabradine has also been used in treating automatic tachycardias such as ectopic atrial tachycardia and junctional ectopic tachycardia [4, 5]. Ivabradine’s cardiac adverse effects include atrial fibrillation, sinus node dysfunction, atrioventricular block, and bundle branch block [1]. We describe a case of ivabradine overdose in a neonate, who was treated for incessant ectopic atrial tachycardia. The patient inadvertently received 10 times the prescribed dose due to inaccurate measurement of the liquid formulation of the drug. The patient also developed sinus bradycardia with heart rate as low as 50 beats per minute (bpm). The patient remained hemodynamically stable. Her heart rate improved and returned to baseline 28 h after discontinuation of ivabradine and other antiarrhythmic agents.
| Case Report | ▴Top |
A full-term infant was found to have a narrow complex tachyarrhythmia at 260 bpm in the delivery room. This episode lasted for 20 min and resolved spontaneously. The tachycardia recurred 3 days later with episodes lasting between 5 and 60 min at heart rate 250 bpm suggestive of ectopic atrial tachycardia on electrocardiogram (Fig. 1a). Adenosine was given and revealed a unifocal ectopic atrial tachycardia. The echocardiogram showed a structurally normal heart with low normal left ventricle systolic function (shortening fraction 27%). The tachycardia was briefly rate controlled with esmolol (Fig. 1b). When rate control was no longer achieved despite high dose of esmolol, treatment first with procainamide (50 µg/kg/min) then with amiodarone (5 mg/kg bolus and 15 mg/kg/day continuous infusion) was unsuccessful. Ivabradine at 0.05 mg/kg/dose (0.22 mg/dose) twice a day was started on day of life 5. After one dose of ivabradine while remaining on the amiodarone infusion of 15 mg/kg/min, her heart rate decreased to 120 bpm, and she intermittently converted to sinus rhythm. From the second dose onward, she remained mostly in sinus rhythm with infrequent and short episodes of atrial tachycardia. The amiodarone was decreased to 5 mg/kg/day. On day of life 9, she was noted to be more bradycardic 2 h following ivabradine dosing with heart rate nadir of 50 bpm at 4 h after the dose (Fig. 2). Review of the event revealed she had inadvertently received an ivabradine dose of 2.2 mg (0.5 mg/kg) instead of the intended dose of 0.22 mg (0.05 mg/kg) due to dosing error. She developed sinus bradycardia with normal PR, QRS and QT intervals for her age (Fig. 3). Amiodarone and ivabradine were discontinued. She remained hemodynamically stable with normal blood pressure and end organ perfusion. Her heart rate returned to baseline after 28 h. Subsequently, she remained in sinus rhythm with fewer episode of atrial tachycardia than at presentation. Once her heart rate returned to baseline, flecainide was initiated, in part due to the overdose and the potential for similar errors as an outpatient. She remained in sinus rhythm on flecainide and was discharged home on day of life 14. Flecainide was discontinued at 6 months of age, and tachycardia had not recurred at last follow-up at 1 year of age.
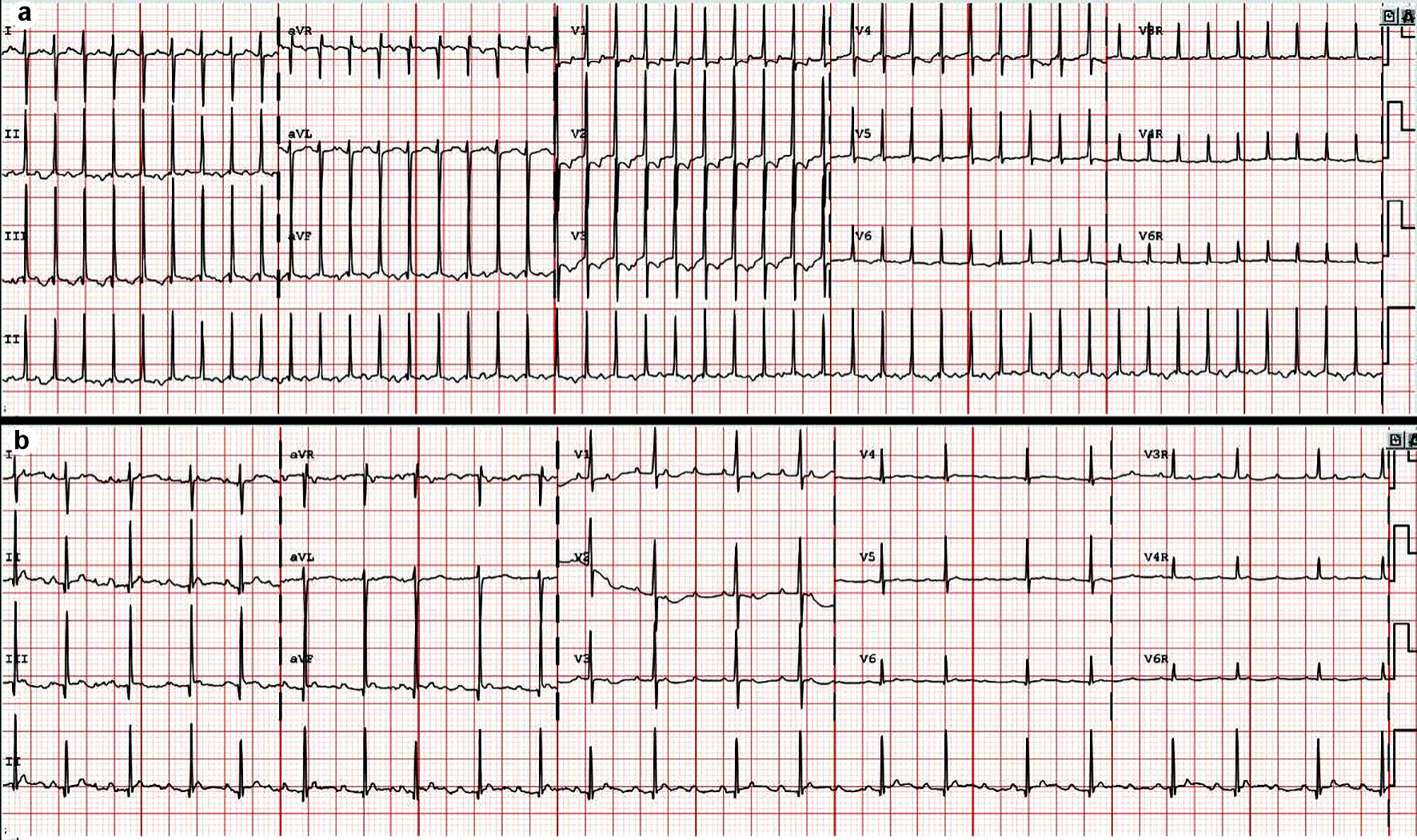 Click for large image | Figure 1. (a) Presenting rhythm on electrocardiogram. Regular narrow complex tachycardia with visible P wave and long PR interval suggestive of ectopic atrial tachycardia. (b) Electrocardiogram on esmolol. Ectopic atrial tachycardia with variable atrioventricular conduction. |
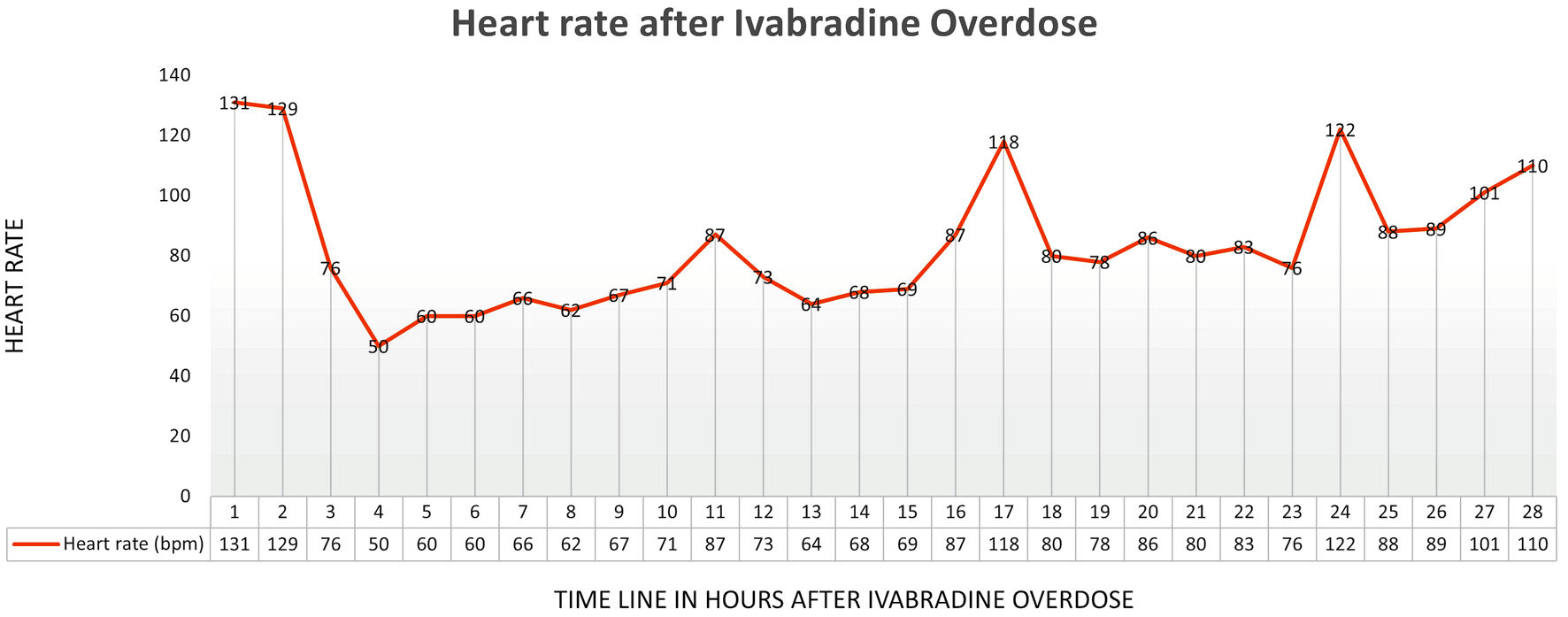 Click for large image | Figure 2. Heart rate trend after ivabradine overdose. |
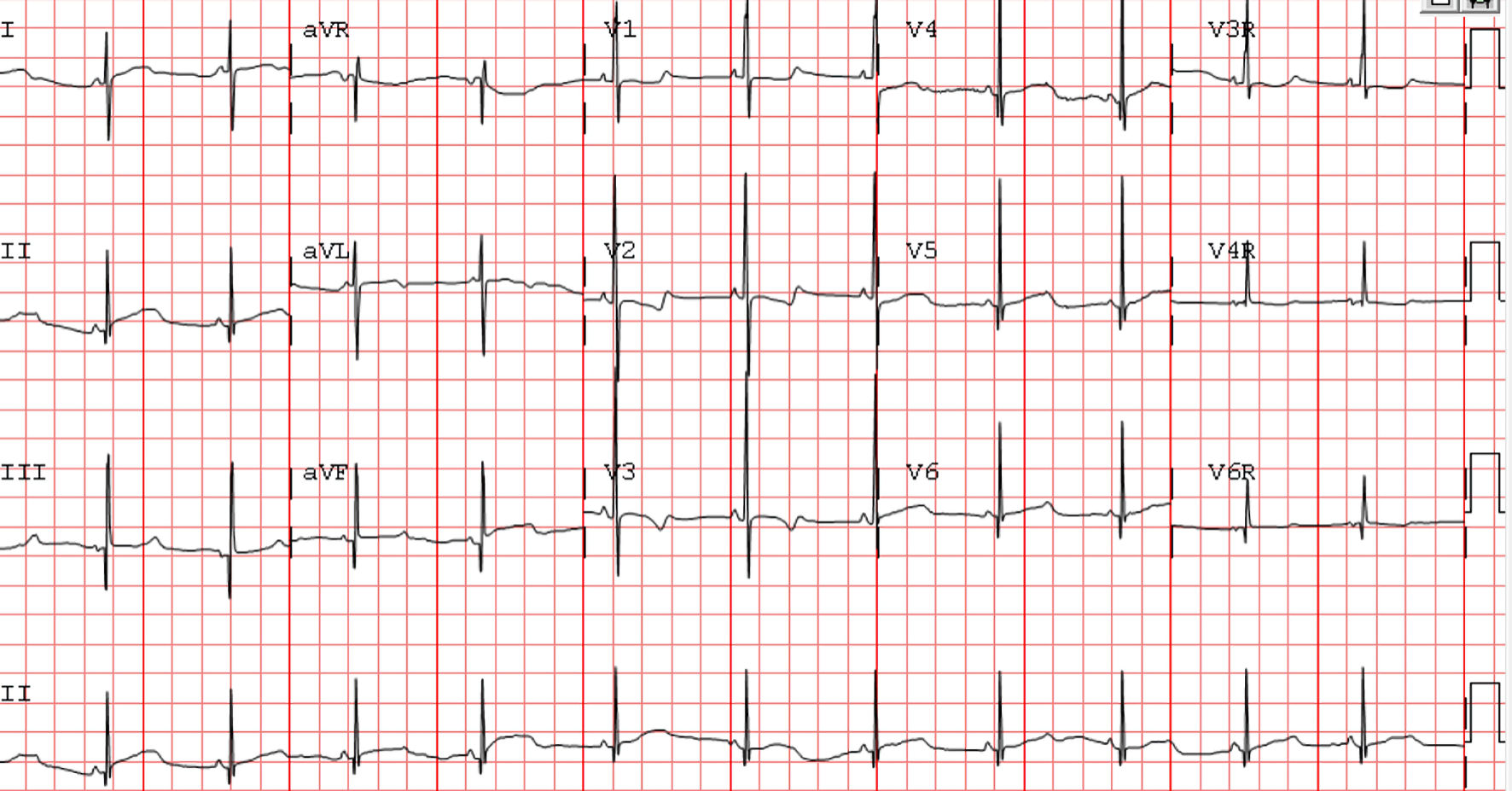 Click for large image | Figure 3. Electrocardiogram after overdose. Sinus bradycardia. Heart rate 70 beats per minute (bpm), PR interval 87 ms, QRS interval 59 ms, and QTc interval 454 ms. |
| Discussion | ▴Top |
Both tablet and solution formulations of ivabradine exhibit rapid absorptive kinetics with peak plasma concentrations at 1 h post enteral administration. In the absence of food, overall bioavailability is estimated to be 40% due to first-pass elimination post absorption in the small intestine. In infants and children, ivabradine is administered with food to optimize the overall plasma exposure. Ivabradine exhibits linear kinetics at therapeutic doses relying on extensive hepatic and intestinal metabolism via CYP3A4 with formation of a potent metabolite with therapeutic equivalence to its parent compound [6]. Both the parent drug and active metabolite are eliminated via renal and fecal excretion. CYP3A4 expression is immature after birth, reaching about 30% to 40% of adult levels by the end of the first month of life; full maturation is not seen until 3 years of age [7]. Overall, Ivabradine’s pharmacokinetic modelling data are lacking in children.
There is a scarcity of literature available describing toxicologic response to ivabradine in humans and none for children. There has been a total of four cases describing ivabradine toxidromes in adult patients [8-11]. The adverse effects of overdose included severe sinus bradycardia with associated cardiogenic shock and sinus pauses up to 11 s [8-11]. Atropine was used in two patients whereas isoproterenol was used in the other two. Of those who were treated with isoproterenol, temporary transvenous pacing was needed as the heart rate rise was inadequate [9, 11]. Adult pharmacodynamic studies suggest that the heart rate lowering effect of ivabradine plateaus at doses exceeding 40 mg/day [6]. Post-mortem serum ivabradine concentration exceeded 1,000 ng/mL in the fatal overdose [12]. Therapeutic concentrations are generally described as approximately 10 - 50 ng/mL and previous case reports have documented non-fatal concentrations up to 525 ng/mL [12]. Overall, toxic doses and associated serum ivabradine concentrations are not yet established.
In our neonate, a dose of 0.5 mg/kg, was associated with significant bradycardia. The heart rate nadir was 4 h after the enteral overdose as expected. She maintained adequate blood pressure and perfusion despite her low heart rate and did not require intervention after discontinuation of the ivabradine and amiodarone. Her heart rate started returned to baseline 28 h after the dose. Interestingly, this high dose was more effective at suppressing the ectopic tachycardia suggesting a dose response relationship previously described in children with heart failure and sinus tachycardia [3]. Pharmacokinetic modeling data indicate that the total clearance increases with increasing age suggesting children must be administered a higher weight-normalized dose to achieve comparable systemic exposure to adults [13]. This phenomenon may explain suppression of ectopic tachycardia at higher dose in this case.
The patient was also on amiodarone that could have further accentuated the bradycardia. On the other hand, amiodarone and ivabradine have different pharmacokinetic and dynamic properties. Ivabradine has previously been used concomitantly with amiodarone to treat junctional ectopic tachycardia at doses 0.02 to 0.1 mg/kg/day with rarely reported severe adverse effects [4, 14]. In small children, precautions are needed when preparing the drug. The lowest concentration commercially available is 1 mg/mL in a 5-mL ampule. Due to lack of stability data from the manufacturer, it is not appropriate to dispense patient-specific doses and thus it falls upon the bedside to appropriately measure a patient-specific dose while inpatient. When a patient is discharged, the ampules are also dispensed, and caregivers are required to withdraw the proper medication volume at home. The measuring and delivery process in infants and children are potential sources of error.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the parent via phone.
Author Contributions
Doctor drafted manuscript, performed literature review. Scott edited the manuscript, reviewed the data and ECGs. Tindell edited the manuscript, performed literature review, reviewed pharmacology data. Nguyen edited the manuscript, performed literature review, reviewed data and ECGs. All authors approved the submission.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Nawarskas JJ, Bowman BN, Anderson JR. Ivabradine: a unique and intriguing medication for treating cardiovascular disease. Cardiol Rev. 2015;23(4):201-211.
doi pubmed - Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376(9744):875-885.
doi - Bonnet D, Berger F, Jokinen E, Kantor PF, Daubeney PEF. Ivabradine in children with dilated cardiomyopathy and symptomatic chronic heart failure. J Am Coll Cardiol. 2017;70(10):1262-1272.
doi pubmed - Arvind B, Kothari SS, Juneja R, Saxena A, Ramakrishnan S, Gupta SK, Chowdhury UK, et al. Ivabradine versus amiodarone in the management of postoperative junctional ectopic tachycardia: a randomized, open-label, noninferiority study. JACC Clin Electrophysiol. 2021;7(8):1052-1060.
doi pubmed - Cohen MI, Cohen JA, Shope C, Stollar L, Collazo L. Ivabradine as a stabilising anti-arrhythmic agent for multifocal atrial tachycardia. Cardiol Young. 2020;30(6):899-902.
doi pubmed - Ivabradine package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/209964lbl.pdf.
- O'Hara K, Wright IM, Schneider JJ, Jones AL, Martin JH. Pharmacokinetics in neonatal prescribing: evidence base, paradigms and the future. Br J Clin Pharmacol. 2015;80(6):1281-1288.
doi pubmed - Mathiaux F, Dulaurent S, Julia F, Gaulier JM. Case report of ivabradine intoxication. J Anal Toxicol. 2014;38(4):231-232.
doi pubmed - Gomez Casal V, Lage Cendon L, Lago Preciado G, Vara Adrio S. [Ivabradine poisoning with suicide intention]. Med Intensiva. 2015;39(9):577-579.
doi pubmed - Maskell K, Tse A, Wolf CE, Troendle M. Acute on chronic ivabradine overdose: a case report. J Med Toxicol. 2016;12(2):189-191.
doi pubmed - Osei K, Taskesen T, Goerbig-Campbell J, Hounshell T. Ivabradine toxicity: A case report and review. HeartRhythm Case Rep. 2020;6(4):183-186.
doi pubmed - Knapp-Gisclon A, Zerah M, Mayer-Duverneuil C, Rambaud C, de la Grandamison GL, Alvarez JC. Fatal intoxication with ivabradine: First case report. Forensic Sci Int. 2020;311:110288.
doi pubmed - Peigne S, Bouzom F, Brendel K, Gesson C, Fouliard S, Chenel M. Model-based approaches for ivabradine development in paediatric population, part I: study preparation assessment. J Pharmacokinet Pharmacodyn. 2016;43(1):13-27.
doi pubmed - Dieks JK, Klehs S, Muller MJ, Paul T, Krause U. Adjunctive ivabradine in combination with amiodarone: A novel therapy for pediatric congenital junctional ectopic tachycardia. Heart Rhythm. 2016;13(6):1297-1302.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


