| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Case Report
Volume 13, Number 4, August 2022, pages 250-254
Serial Changes in Troponin I in COVID-19 Vaccine-Associated Myocarditis
Jairo Aldana-Bitara, b, g , Noah R. Ramireza, Allan S. Jaffec, d, Venkat S. Manubolub, Dhiran Vergheseb, Luay Husseinb, Lauren R. Andersona, Matthew J. Budoffb, Ronald P. Karlsberga, e, f
aCardiovascular Research Foundation of Southern California, Beverly Hills, CA, USA
bThe Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center, Los Angeles, CA, USA
cDepartment of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA
dDepartment of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, USA
eCedars-Sinai Smidt Heart Institute, Los Angeles, CA, USA
fDavid Geffen School of Medicine at UCLA, Los Angeles, CA, USA
gCorresponding Author: Jairo Aldana-Bitar, Cardiovascular Research Foundation of Southern California, Beverly Hills, CA 90210, USA
Manuscript submitted July 21, 2022, accepted July 29, 2022, published online August 15, 2022
Short title: COVID-19 Vaccine and Myocarditis
doi: https://doi.org/10.14740/cr1412
| Abstract | ▴Top |
A 63-year-old woman presented with atypical chest pain after a third dose of the coronavirus disease 2019 (COVID-19) messenger ribonucleic acid (mRNA) vaccine. Serial cardiac troponin measurements were performed to evaluate the trajectory of her time-concentration curve which showed a typical myocarditis curve with rapid normalization. The diagnosis of myocarditis was confirmed by cardiac magnetic resonance imaging and follow-up imaging showed resolution. All symptoms resolved with weeks.
Keywords: Myocarditis; COVID-19; Vaccine; Troponin curve
| Introduction | ▴Top |
The Food and Drug Administration (FDA) has approved four vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by July 2022 [1]. Side effects from the vaccines are rare, and one of them is myocarditis [2]. The diagnosis of myocarditis is usually made with the use of cardiac imaging, such as cardiac magnetic resonance imaging (MRI) [3], which is an important tool for confirming the diagnosis; however, the use of troponin is part of the initial workup for the patients with suspected myocarditis and only few cases have reported the troponin curve from the beginning of symptoms until the resolution of the disease. Here, we report a case with time-concentration serial changes of troponin I from SARS-CoV-2 vaccination until the resolution of symptoms, with confirmation and follow-up resolution documented with cardiac MRI.
| Case Report | ▴Top |
Investigations
A 63-year-old woman with a past medical history of rheumatoid arthritis and mixed connective tissue disease presented to the emergency room (ER) with new onset chest pain 11 h following a third dose of the Pfizer BioNTech coronavirus disease 2019 (COVID-19) vaccination. Her physical examination was unremarkable without the findings of pericardial rub or heart murmur.
The patient denied current use of immunosuppressive agents secondary to her autoimmune history at the time of COVID-19 vaccination-induced myocarditis. Of note, the patient was infected with the novel coronavirus in March 2020, with a mild presentation, and was treated with conservative measures and symptom management.
Diagnosis
Differential diagnoses included myocardial infarction (MI), acute coronary syndrome (ACS), and myocarditis. Initial diagnostic work revealed a normal electrocardiogram (ECG) without ST changes and a normal cardiac troponin I value of 0.02 ng/mL (Beckman Coulter, AccuTnI+3, upper reference limit (URL) < 0.03 ng/mL). The following day, she re-presented with persistent chest pain and her cardiac troponin I had risen to 1.60 ng/mL. A second ECG was taken and remained unchanged. Cardiac computed tomography (CT) showed normal coronary arteries, normal left ventricular function, without regional wall motion abnormalities and normal pericardium. A transthoracic echocardiogram revealed neither abnormalities nor pericardial disease. Serial cardiac troponin measurements were subsequently performed to evaluate the trajectory of her time-concentration curve (Fig. 1). Cardiac MRI was performed 11 days post-vaccination and showed abnormal T1 and T2 sequences, an elevated extracellular volume (ECV) in the septum, mid-myocardial late gadolinium enhancement in the basal anteroseptal, inferoseptal, and inferolateral wall segments suggesting focal myocardial edema and fibrosis (Fig. 2). This pattern was interpreted to be most consistent with myocarditis.
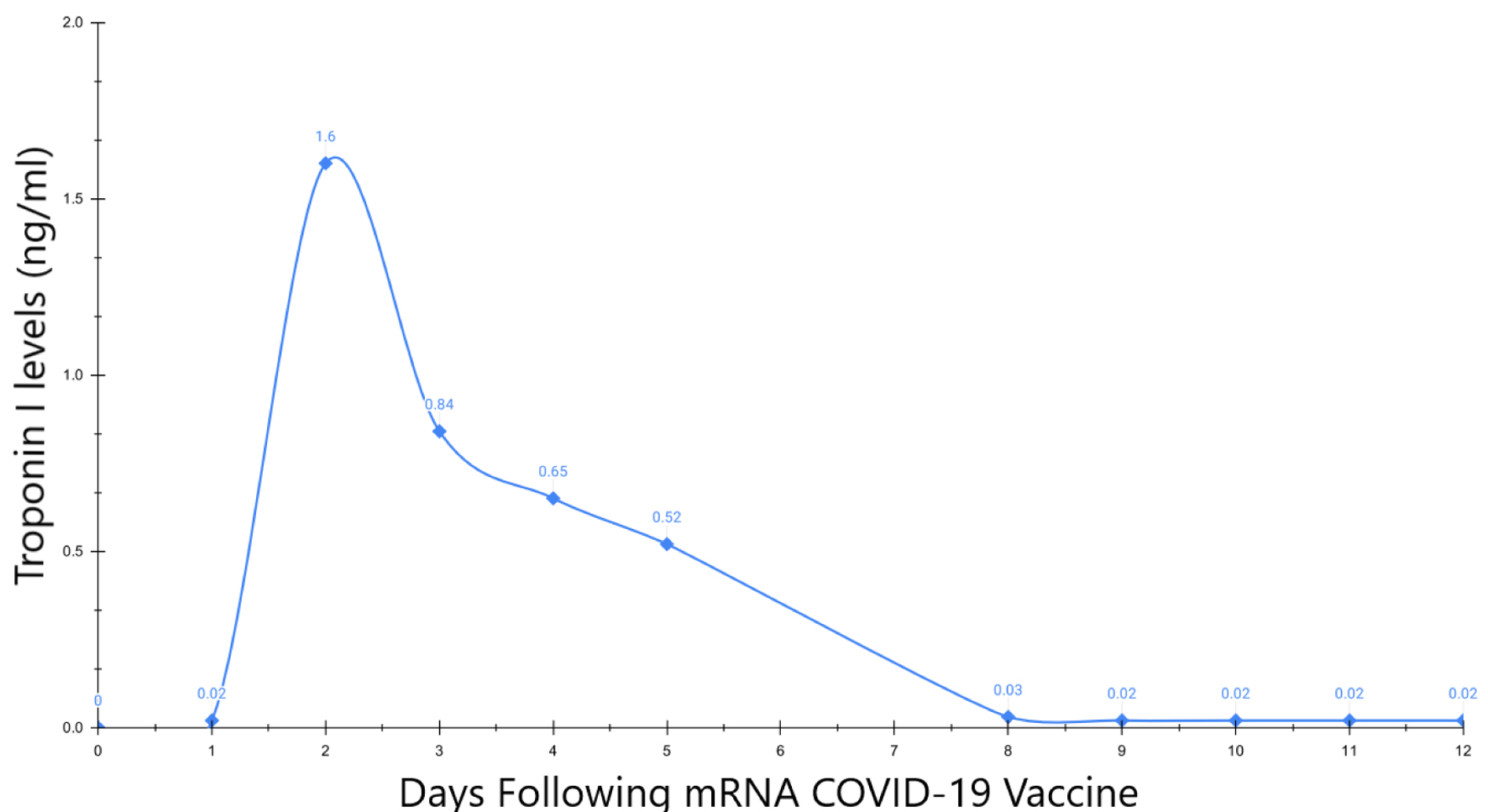 Click for large image | Figure 1. Cardiac troponin I time-concentration curve. |
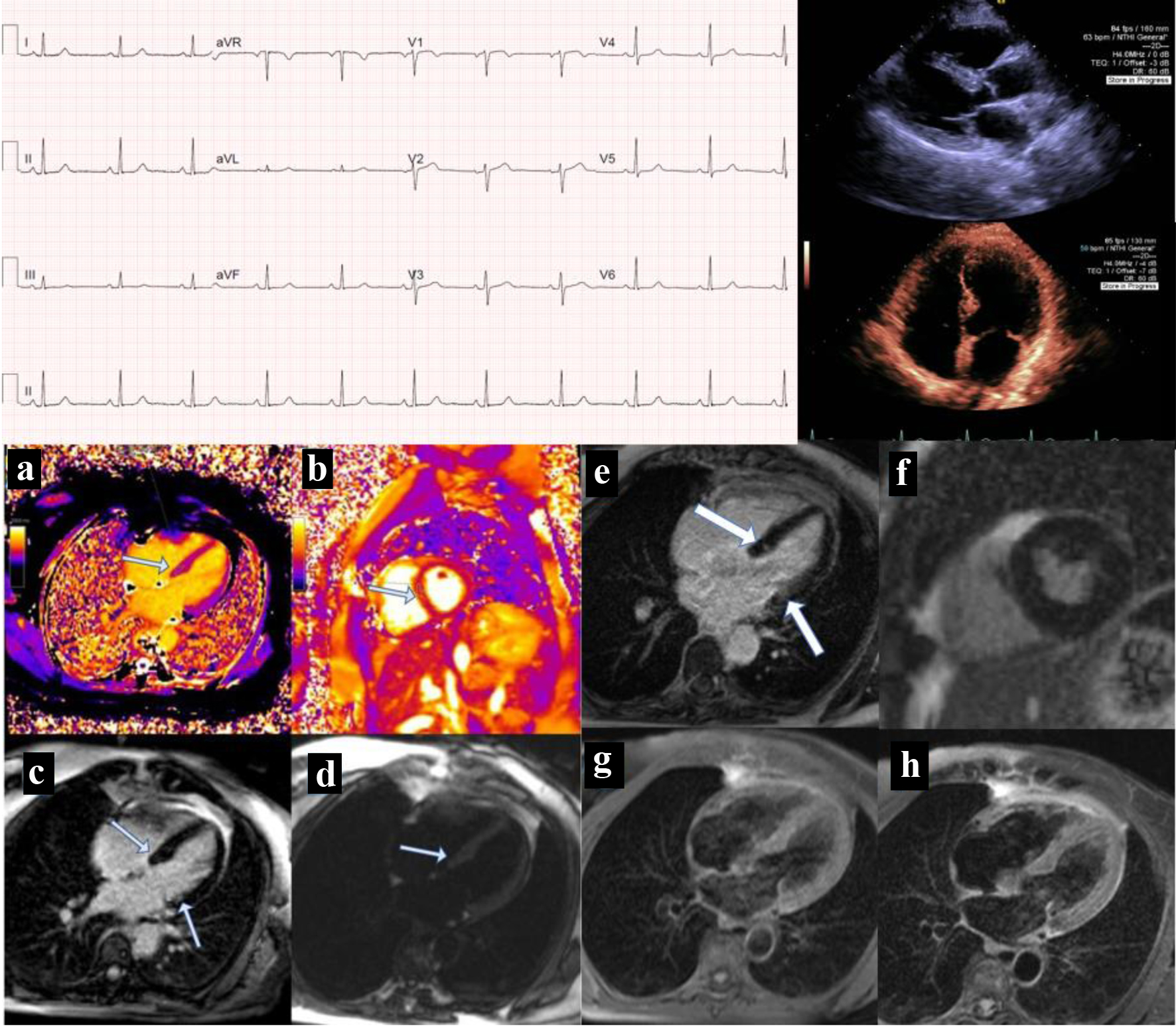 Click for large image | Figure 2. Electrocardiogram and imaging findings. |
Troponin I time-concentration curve is displayed, demonstrating a rise in troponin I levels during the second day post-vaccination followed by a progressive decline and eventual normalization of troponin levels on day 12. After day 12, there were not any detectable levels of the troponin enzyme. This troponin curve is consistent with myocarditis.
ECG shows sinus rhythm with no additional findings and was interpreted as normal. The long parasternal view of the echocardiogram shows normal function and no pericardial disease. The four-chamber view of the echocardiogram demonstrates normal wall motion and no structural heart disease. The patient’s initial cardiac MRI on the bottom left (Fig. 2a-d) reflects a 11-day post-vaccination status. The figure demonstrates (Fig. 2a) T1 mapping, and in this case, its value for the septum was elevated; in Figure 2b, native T2 mapping was elevated which was compatible with edema, on delayed gadolinium enhancement imaging (Fig. 2c). This is shown with mid-myocardial late gadolinium enhancement in the basal anteroseptal, inferoseptal and inferolateral walls, and Figure 2d shows T2-weighted image with edema, and all MRI findings were compatible with myocarditis. The patient’s 9-month follow-up cardiac MRI (Fig. 2e-h) demonstrates (Fig. 2e) late gadolinium enhancement in the basal inferoseptal and anterolateral wall (arrows show regions of interest). The MRI shows resolution of edema and scarred fibrosis. Late gadolinium enhancement short axis imaging (Fig. 2f) mid-portion shows no delayed enhancement or fibrosis. The four-chamber view (Fig. 2g) demonstrates T1-weighted imaging with no hypertense areas and T2-weighted image (Fig. 2h) shows no residual edema.
Treatment
The patient was prescribed a 2-month course of colchicine 0.6 mg twice daily starting 8 days status post the third dose of the vaccination. Additionally, a short course of methyl prednisone was initiated 26 days after the third dose of the vaccination for a 6-day treatment with marked reduction in her chest pain syndrome. No non-steroidal anti-inflammatory drugs (NSAIDs) were given for treatment. Nine months later, follow-up cardiac MRI showed resolution of findings suggestive of myocarditis and there was persistent late gadolinium enhancement in the basal inferoseptal and anterolateral wall.
Follow-up and outcomes
She experienced intermittent, self-resolving episodes of pleuritic chest pain over the subsequent 6 months. No myocardial injury was documented after the myocarditis episode and follow-up cardiac MRI showed resolution of myocarditis findings.
| Discussion | ▴Top |
Myocarditis is an infrequent cardiovascular condition that can arise secondary to COVID-19 vaccination [2]. The incidence of myocarditis from the COVID-19 vaccination has been estimated to be 0.00213% (2.13 per 100,000 persons). The highest incidence of myocarditis appears to be in young men with a rate of 0.01069%. The ages of the patients in the report ranged from 16 to 29 and each had received at least one dose of an mRNA vaccine [4]. The pathophysiology of vaccine-induced myocarditis has been hypothesized to hinge on molecular mimicry. Molecular mimicry occurs when the immune-inducing antigen from the vaccine shares structural similarities with binding epitopes found in the myocardium. As a result, lymphocytes in the myocardium activate, creating inflammation and myocyte injury [5].
In a study of 15 patients, aged 17 - 52 with putative myocarditis, 13 had elevated troponin I levels after receiving a COVID-19 vaccine. The use of the Pfizer BioNTech COVID-19 vaccine was associated with 60% of the cases, 33% arose after Moderna vaccination, and 7% occurred after Johnson & Johnson vaccine [6].
In a separate study, 15 children, aged 12 - 18, were hospitalized at Boston Children’s Hospital with myocarditis after receiving the mRNA COVID-19 vaccine. Each patient had experienced chest pain for 1 - 9 days after receiving the vaccine. The 15 patients had elevated troponin levels with a median value of 0.25 ng/mL and a range from 0.08 to 3.15 ng/mL. After discharge, all patients exhibited troponin levels that were decreased yet still elevated above baseline. In the short term, cases of myocarditis from the vaccine seem to be minor. However, further research is needed to understand and evaluate the long-term effects of COVID-19 vaccine-associated cases of myocarditis [7].
In a study of 809 patients below the age of 30 who were diagnosed with myocarditis after receiving an mRNA vaccine, 97.9% had elevated troponin I levels. These results reflect the critical diagnostic role of cardiac troponin for this diagnosis [8]. According to the Centers for Diseases Control and Prevention (CDC), out of the 296 million doses of Pfizer BioNTech and Moderna COVID-19 vaccination, there have been 1,226 cases of myocarditis, yielding a rate of 4.1 incidents per million doses [9].
Most of these studies do not indicate whether myocarditis occurred after a first, second, or third dose. In one study, seven male adolescents aged between 14 and 19 years experienced myocarditis 4 days after receiving the Pfizer BioNTech vaccine. Three of the seven patients exhibited elevated troponin levels ranging from 2.59 to 22.1 ng/mL [10].
The most common treatment reported for COVID-19 vaccine-related myocarditis is the use of NSAIDs (87%), followed by either intravenous immunoglobulin or glucocorticoid, each used in 12% of cases [8].
In our case, a 63-year-old woman presented with chest discomfort 11 h following a third dose of the Pfizer BioNTech COVID-19 vaccination. The patient’s troponin I levels were then measured serially. As shown by the cardiac troponin I time-concentration curve, the first day after her vaccination, her cardiac troponin I level was normal (0.02 ng/mL) (Fig. 1). The following day, it rose to 1.60 ng/mL and then slowly declined, showing a typical curve of troponin in myocarditis. The decline in her cardiac troponin I levels slowly decreased over the following week. Thereafter, she presented with intermittent chest pain and her symptoms were not correlated to troponin measurements, suggesting a non-cardiac etiology for her chest pain. We are reporting a cardiac troponin time-concentration curve after COVID-19 vaccination, suggesting that troponin changes track well with the initial injury. Notable ECG changes were not found in this case. Colchicine was used for treatment in our case, this drug has several mechanisms of action, it binds to tubulin affecting mitosis, leucocyte movement, exocytosis and phagocytosis, it inhibits the expression of selectins leading to a decreased adherence of leucocytes to the inflamed endothelium [11], and it inhibits NLRP3 inflammasome [12]. Colchicine has been used as part of SARS-CoV-2 myocardial injury treatment [13] and COVID-19 vaccine-related myocarditis, being a common treatment among the cases reported [14]. Another retrospective study showed that in patients with COVID-19 vaccine myocarditis, 48% were treated with colchicine, 35% with aspirin, 20% with ibuprofen, and 5% with steroids [15]. The treatment with colchicine is one of the treatments available among various options for myocarditis [16].
Conclusion
It is well documented that mRNA COVID-19 vaccines are associated with rare incidence of myocarditis. The diagnostic use of cardiac troponin levels is crucial in most of these cases. The cardiac troponin I kinetics in our case tracked with the patient’s initial symptoms and imaging confirmed the presence and subsequent resolution of myocarditis. Further research is required to determine the mechanism of cellular injury that leads to cardiac troponin release after mRNA COVID-19 vaccinations to determine if the rise and disappearance of troponin provides clues to the mechanisms of damage and the course of the disease.
Learning points
This case reports a troponin time-concentration curve after COVID-19 mRNA booster myocarditis.
1) COVID-19 vaccination is increasing worldwide and unwanted side effects occur, such as myocarditis.
2) Troponin measurement is diagnostic for myocardial cell injury and thus the rate of troponin release and normalization may be useful in the diagnosis, and management of COVID-19 vaccine-related myocarditis.
Acknowledgments
Chamath Palihapititiya Post-Graduate Cardiology Fellowship Grant to the Cardiovascular Research Foundation of Southern California.
Financial Disclosure
No funding to declare for this case report.
Conflict of Interest
Dr. Allan Jaffe has or presently consults for most of the major diagnostic companies that make cardiac troponin assays, including the one used in this report. Dr. Matthew Budoff receives support from General Electric (GE) and National Institutes of Health (NIH). No funding or research support was received for this project.
Informed Consent
Informed consent was obtained.
Author Contributions
Dr. Jairo Aldana-Bitar structured the paper, wrote the abstract and the introduction, analyzed the images and the troponin curve, and reviewed the entire document. Noah R. Ramirez wrote part of the discussion, did the troponin curve image and reviewed the entire document. Dr. Allan Jaffe did edits for the entire document, participated doing the research of troponin curve and reviewed the entire document. Dr. Venkat S. Manubolu, Dr. Dhiran Verghese, and Dr. Luay Hussein did part of research on the troponin concentration curve information, and reviewed the entire document. Lauren R. Anderson wrote part of the investigations, diagnosis and treatment and reviewed the entire document. Dr. Matthew J. Budoff and Ronald P. Karlsberg guided the team in how to structure the paper, edited/revised statements reviewed and approved the entire document.
Data Availability
The authors declare that data supporting the finding of this case report are available within the article.
Abbreviations
mRNA: messenger ribonucleic acid; ECG: electrocardiogram; CT: computed tomography; ECV: extracellular volume; MRI: magnetic resonance imaging; CDC: Centers for Diseases Control and Prevention; FDA: Food and Drug Administration
| References | ▴Top |
- COVID-19 Vaccines. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines.
- Shiravi AA, Ardekani A, Sheikhbahaei E, Heshmat-Ghahdarijani K. Cardiovascular complications of SARS-CoV-2 vaccines: an overview. Cardiol Ther. 2022;11(1):13-21.
doi pubmed - Friedrich MG, Marcotte F. Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging. 2013;6(5):833-839.
doi pubmed - Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, Grinberg T, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139.
doi pubmed - D'Angelo T, Cattafi A, Carerj ML, Booz C, Ascenti G, Cicero G, Blandino A, et al. Myocarditis after SARS-CoV-2 vaccination: a vaccine-induced reaction? Can J Cardiol. 2021;37(10):1665-1667.
doi pubmed - Salah HM, Mehta JL. COVID-19 vaccine and myocarditis. Am J Cardiol. 2021;157:146-148.
doi pubmed - Dionne A, Sperotto F, Chamberlain S, Baker AL, Powell AJ, Prakash A, Castellanos DA, et al. Association of myocarditis with BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA Cardiol. 2021;6(12):1446-1450.
doi pubmed - Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, Edwards K, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340.
doi pubmed - Pepe S, Gregory AT, Denniss AR. Myocarditis, pericarditis and cardiomyopathy after COVID-19 vaccination. Heart Lung Circ. 2021;30(10):1425-1429.
doi pubmed - Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, Collins JS, Shaughnessy R, et al. Symptomatic Acute Myocarditis in 7 Adolescents After Pfizer-BioNTech COVID-19 Vaccination. Pediatrics. 2021;148(3):e2021052478.
doi pubmed - Imazio M, Andreis A, Brucato A, Adler Y, De Ferrari GM. Colchicine for acute and chronic coronary syndromes. Heart. 2020;106(20):1555-1560.
doi pubmed - Martinez GJ, Celermajer DS, Patel S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis. 2018;269:262-271.
doi pubmed - Anton-Vazquez V, Byrne L, Anderson L, Hamzah L. COVID-19 cardiac injury and the use of colchicine. BMJ Case Rep. 2021;14(2):e241047.
doi pubmed - Fatima M, Ahmad Cheema H, Ahmed Khan MH, Shahid H, Saad Ali M, Hassan U, Wahaj Murad M, et al. Development of myocarditis and pericarditis after COVID-19 vaccination in adult population: A systematic review. Ann Med Surg (Lond). 2022;76:103486.
doi pubmed - Fronza M, Thavendiranathan P, Chan V, Karur GR, Udell JA, Wald RM, Hong R, et al. Myocardial injury pattern at MRI in COVID-19 vaccine-associated myocarditis. Radiology. 2022:212559.
doi pubmed - Tschope C, Cooper LT, Torre-Amione G, Van Linthout S. Management of myocarditis-related cardiomyopathy in adults. Circ Res. 2019;124(11):1568-1583.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


