| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 14, Number 2, April 2023, pages 115-122
Mechanism of Increased Spinal Cord Blood Flow due to Noradrenaline Administration Using Vascular Resistance: An Experimental Study Using a Canine Model
Yuya Kisea, c, Yukio Kuniyoshib, Keita Miyaishia, Mizuki Andoa, Shotaro Higaa, Tatuya Maedaa, Moriyasu Nakaemaa, Hitoshi Inafukua, Kojiro Furukawaa
aDepartment of Thoracic and Cardiovascular Surgery, Graduate School of Medicine, University of the Ryukyus, Okinawa, Japan
bDepartment of Cardiovascular Surgery, Urasoe General Hospital, Urasoe, Okinawa, Japan
cCorresponding Author: Yuya Kise, Department of Thoracic and Cardiovascular Surgery, Graduate School of Medicine, University of the Ryukyus, Nishihara, Okinawa 903-0215, Japan
Manuscript submitted February 1, 2023, accepted March 10, 2023, published online April 8, 2023
Short title: Increased SCBF due to Noradrenaline
doi: https://doi.org/10.14740/cr1478
| Abstract | ▴Top |
Background: During thoracoabdominal aortic surgery, the spinal cord is placed under ischemic conditions. Elevation of systemic blood pressure is thus recommended as a method of increasing the blood supply from collateral networks. This study examined the mechanisms by which noradrenaline administration increases spinal cord blood flow (SCBF) by elevating systemic blood pressure.
Methods: In beagles (n = 7), the thoracoabdominal aorta and L2-L7 spinal cord segmental arteries (SAs) were exposed and a distal perfusion bypass was created to simulate clinical practice. SCBF was measured by laser flowmetry at the L5 dura mater and spinal cord perfusion pressure (SCPP) was measured inside the clamped aorta. The six pairs of SAs from L2 to L7 were clamped, and mean systemic blood pressure (mSBP), SCBF, and SCPP were measured before and after clamping and after starting continuous infusion of noradrenaline at 0.5 µg/kg/min. Rates of change in systemic vascular resistance (SVR) and spinal cord vascular resistance (SCVR) were calculated from the measured values.
Results: With no SA clamping (control), the rate of increase in SCVR was 0.74 times the rate of increase in SVR (y = 0.2 + 0.74x, r = 0.889, r2 = 0.789; P < 0.01). When all six pairs of SAs were clamped, a weak correlation was evident between rate of change in SCVR and rate of change in SVR, and the rate of increase in SCVR was lower than the rate of increase in SVR (y = 0.39 + 0.07x, r = 0.209, r2 = 0.039; P < 0.01). When all six pairs of SAs were clamped in the absence of distal perfusion, a weak correlation was also evident between rate of change in SCVR and rate of change in SVR, and the rate of increase in SCVR was lower than the rate of increase in SVR (y = 0.19 + 0.08x, r = 0.379, r2 = 0.144; P < 0.01).
Conclusions: The rate of increase in SCVR induced by noradrenaline administration was lower than the rate of increase in SVR in the control group with no spinal cord SA clamping and in both experimental groups with clamped SAs (with and without distal perfusion), creating an environment conducive to spinal cord flow distribution.
Keywords: Spinal cord vascular resistance; Spinal cord blood flow; Thoracoabdominal aortic aneurysm; Segmental arterial pressure
| Introduction | ▴Top |
During thoracoabdominal aortic aneurysm surgery with extensive segmental artery (SA) clamping, antegrade perfusion from the SAs decreases. As a result, spinal cord blood flow (SCBF) becomes dependent on the supply of blood via collateral networks. In this environment, increasing the systemic blood pressure represents an effective means of increasing collateral flow to the spinal cord. In particular, maintaining mean systemic blood pressure (mSBP) at ≥ 80 mm Hg has been reported to decrease the occurrence of spinal cord ischemic injury (SCII) [1-5]. Fluid loading or administration of β-agonists such as dopamine and dobutamine both increase cardiac output (CO) and promote systemic blood pressure elevation, so the fact that this leads to increased SCBF via collateral networks is readily understandable. In actual clinical practice, however, vasopressors that act on α-adrenoreceptors, such as noradrenaline and phenylepinephrine, are widely used to achieve sufficient elevations in systemic blood pressure [1, 4]. The problem with using noradrenaline is that because this agent acts primarily on α1-adrenoreceptors, increased vascular resistance in the affected organs may lead to decreased blood flow in those organs (in this case, diminished SCBF). As a result, no clear conclusion has yet been reached concerning whether noradrenaline should be used to increase SCBF during thoracoabdominal aortic aneurysm surgery.
In neurosurgery and orthopedic surgeries, however, the use of noradrenaline to maintain organ perfusion pressure (ΔP) and organ flow (OF) in the brain and spinal cord in patients with traumatic brain or spinal cord injury is widely recommended [6-8]. We have previously conducted acute-phase experiments in beagles to simulate thoracoabdominal aortic surgery, revealing a strong positive correlation between the rise in systemic blood pressure when noradrenaline was administered and the increase in SCBF in both SA-clamped and non-SA-clamped groups [9]. In the present study, we elucidated the mechanisms by which administration of noradrenaline increases SCBF by elevating mSBP.
Factors determining mSBP and OF
The calculation of mSBP from CO and systemic vascular resistance (SVR), as independent variables, is achieved using the following formula: mSBP = CO × SVR
OF is also calculated using ΔP and organ vascular resistance (OVR) as independent variables, as expressed by the following formula: OF = ΔP/OVR
Pharmacological mechanism of action for noradrenaline
Noradrenaline acts mainly on α1 receptors to elevate blood pressure by increasing SVR. Because CO remains unchanged or is only reduced slightly [10, 11], the rate of change in mSBP approximates the rate of change of SVR: mSBP (↑) = CO (→) × SVR (↑); Rate of change of mSBP ≈ rate of change of SVR
CO and OF
CO represents the total of distributed OFs for each organ (OF1 + OF2 + OF3 + … OFn): CO = OF1 + OF2 + OF3 + … OFn.
Hypothesis
Following noradrenaline administration, CO is generally stable, and the amount of blood from this output that is distributed to each organ is determined by the vascular resistance of that organ. That is, organs with higher vascular resistance receive lower flow, while those with lower vascular resistance receive higher flow. Because the rate of increase in the vascular resistance of each organ (vascular responsiveness) as a result of noradrenaline varies, the amount of flow distributed to each organ is determined by these differences in the rate of increase (rate of change) in vascular resistance.
Following noradrenaline administration, CO is generally stable, because the elevated SVR represents the sum of the vascular resistances for all individual organs: 1) if the rate of increase in vascular resistance in organ α (OVRα) is greater than the rate of increase in the SVR (as the sum of vascular resistance in all organs), then blood flow to organ α will decrease; and conversely, 2) if the rate of increase in OVRα is less than the rate of increase in SVR, blood flow to organ α will increase.
1) Rate of increase in OVRα > rate of increase in SVR (sum of vascular resistances of all individual organs) → OFα (OF to organ α) decreases.
2) Rate of increase in OVRα < rate of increase in SVR → OFα increases.
On the basis of these assumptions, we hypothesized that the mechanism by which SCBF is increased as a result of the increase in systemic blood pressure induced by noradrenaline administration is that the rate of increase in SCVR is lower than the rate of increase in SVR, with flow distribution to the spinal cord increasing accordingly. In this study, we compared the rates of increase in SCVR and SVR induced by noradrenaline administration, and demonstrated that the rate of increase in SCVR is lower than the rate of increase in SVR. We also compared the increases in SCVR and SVR in a distal perfusion model with extensive spinal cord SA clamping, reflecting actual clinical practice, and in a distal non-perfusion model.
SCVR was derived from spinal cord perfusion pressure (SCPP) and SCBF: SCVR = SCPP/SCBF
The rate of change compared with pre-noradrenaline administration as the baseline was expressed by the following formula: Rate of change in SCVR = rate of change in SCPP/rate of change in SCBF
Because CO is almost constant during noradrenaline administration, the rate of change in SVR can be approximated as the rate of change in mSBP: Rate of change in SVR ≈ rate of change in mSBP
| Materials and Methods | ▴Top |
We used our previously developed beagle model, in which six sets of spinal SAs (including the artery of Adamkiewicz (AKA)) were clamped, in an experimental setup that enabled the measurement of mSBP, SCPP, and SCBF, and recorded these measurements during noradrenaline administration.
The experimental method is described below [9].
Animals
Animal care and all procedures were performed in compliance with the Guide for Care of Laboratory Animals. This study was approved by the Research Committee for Laboratory Animal Science at the University of Ryukyus, Japan. Experiments were performed on seven female beagle dogs weighing 7.5 - 10.0 kg.
Anesthesia
Dogs were sedated by intramuscular injection of ketamine hydrochloride at 3 mg/kg and 0.2 mg of atropine sulfate. Dogs were then intubated endotracheally and ventilated mechanically. One intravenous line was inserted into an anterior limb vein. Dogs were placed in a prone position on an operating table under intravenous anesthesia with propofol (0.3 mg/kg/min) and ketamine hydrochloride (0.05 mg/kg/min).
During the surgical procedure, mSBP was maintained between 70 and 100 mm Hg by controlling the propofol concentration and fluid infusion. By monitoring with a rectal temperature probe, body temperature was maintained between 36 and 37 °C using a heating pad and blanket. Arterial blood gases were measured (i-STAT1®; FUSO Pharmaceutical, Osaka, Japan) at 60-min intervals. The metabolic and respiratory acid-base balance was confirmed to be maintained between 7.35 and 7.45 pH, PaO2 was maintained above 100 mm Hg, and PaCO2 was maintained between 35 and 45 mm Hg by adjusting the respiratory volume and rate.
Surgical procedure
Surgery was performed by a single cardiovascular surgeon to exclude effects on measurements related to differences in surgeon expertise.
First, L4 laminectomy was performed, and a 1.5 × 1.5 cm area of the dorsal aspect of the dura mater over the spinal cord was exposed.
Second, dogs were placed in the right decubitus position. The chest was opened through a thoracotomy in the ninth left intercostal space. The abdominal aorta was exposed through a left flank incision and exfoliated from the descending aorta (at the Th11 level) to the trifurcation, with careful exposure of the L2-L7 SAs. After heparin was administered intravenously at 200 IU/kg, a temporary descending aorta-to-left external iliac artery bypass was created. A 10-Fr aortic cannula (Duraflo II; Edwards Lifesciences, Irvine, CA) was inserted at proximal sites, an 8-mm woven graft was anastomosed at distal sites, and these devices were connected.
Next, 22-G cannulae were placed at the left common carotid artery, right femoral artery, and L5 level abdominal aorta to monitor mean proximal arterial blood pressure (mPAP), mean distal arterial blood pressure (mDAP), and SA pressure (SAP), respectively.
SCPP is generally expressed as SAP - cerebrospinal fluid pressure (CSFP). However, as we were unable to measure CSFP in this experiment due to technical problems. CSFP was therefore assumed to be a uniform 6 mm Hg, as previous studies have shown that mean CSFP in beagle dogs is 6 mm Hg [12]. The SAP - 6 mm Hg was substituted for SCPP while the abdominal aorta was clamped (between L3 to L4 and L6 to L7) (Fig. 1). Since AKA branches from the L4 or L5 SA in dogs, this range of interruption included the AKA [13]. The SCPP under the control condition in which the aorta was not clamped was expressed as mSBP - 6 mm Hg.
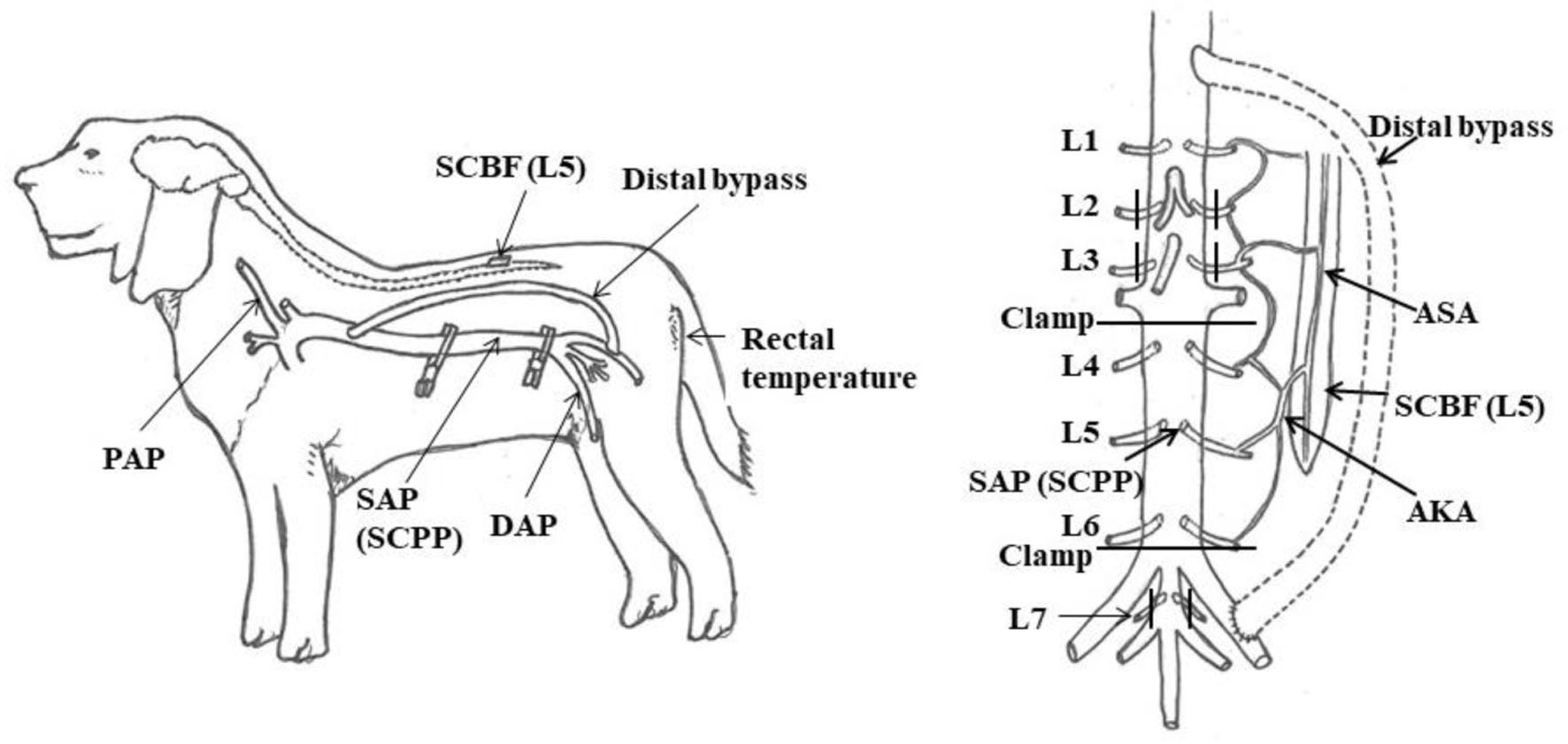 Click for large image | Figure 1. Experimental model. AKA: artery of Adamkiewicz; ASA: anterior spinal artery; DAP: distal arterial blood pressure; PAP: proximal arterial blood pressure; SAP: segmental arterial pressure; SCBF: spinal cord blood flow; SCPP: spinal cord perfusion pressure. Reproduced from Journal of Thoracic and Cardiovascular Surgery, in press [9]. ©Elsevier. |
Measurement of SCBF
SCBF was measured using laser Doppler flowmetry (Omegaflow FLO-N1; Neuroscience, Tokyo, Japan) to assess real-time microcirculatory changes in the spinal cord. The laser probe was placed in contact with the intact dorsal dura mater at the L5 segmental level of the spinal cord, which was at the upper L4 vertebral level and connected to the laser Doppler flowmeter. The probe was affixed in a riding position over the spinal cord. Output signals were collected continuously throughout the experiment and averaged every 3 s.
Experimental protocol
Mean blood pressures (mPAP, mDAP, and SAP (SCPP)) and SCBF were measured at the same time under the following three conditions (Fig. 2): condition 1, no aortic clamp and no SA clamp (control group); condition 2, aortic clamping (between L3 to L4 and L6 to L7), and L2, L3, and L7 SA clamps (L2-L7 SA flow halted) with distal perfusion; and condition 3, aortic clamping (between L3-L4 and L6-L7) and L2, L3, and L7 SA clamps (L2-L7 SA flow halted) with no distal perfusion.
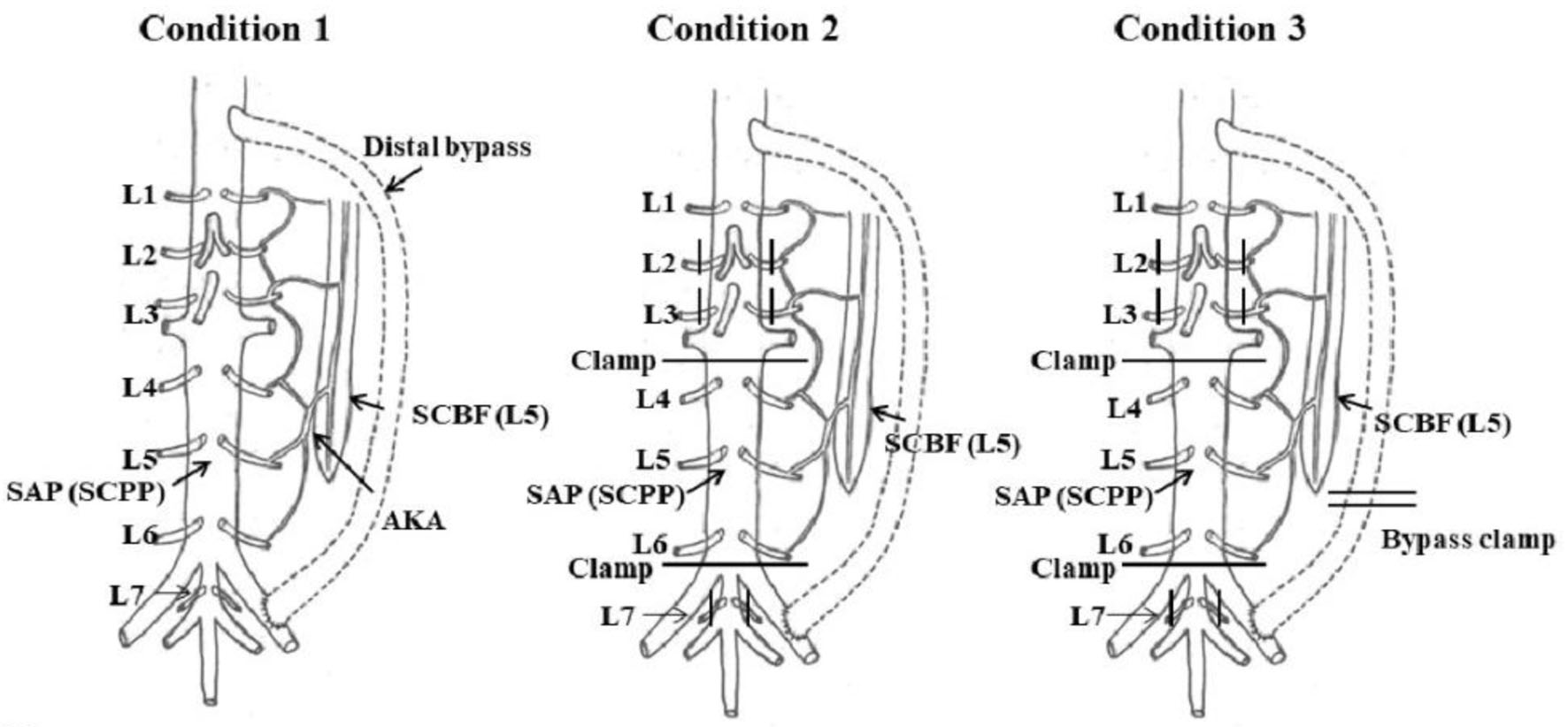 Click for large image | Figure 2. Experimental protocol. Condition 1: no aortic clamp and no SA clamp (control group). Condition 2: L2-L7 SA flow halted with distal perfusion. Condition 3: L2-L7 SA flow halted with no distal perfusion. AKA: artery of Adamkiewicz; SAP: segmental arterial pressure; SCBF: spinal cord blood flow; SCPP: spinal cord perfusion pressure. Reproduced from Journal of Thoracic and Cardiovascular Surgery, in press [9]. ©Elsevier. |
Mean values for each measurement over 5 min from the start of measurements were taken as baseline values. After completing baseline measurements, condition 1 was created, and continuous administration of 0.5 µg/kg/min noradrenaline was started. Measurement data for each site were sampled every 15 s from the start of the rise in mSBP (taking the mPAP measurement value as mSBP). Continuous administration of noradrenaline was stopped after 10 min, and data sampling was performed for about 10 min as blood pressure naturally decreased and stabilized. The same procedure was continued for conditions 2 and 3.
A difference of less than 10 mm Hg between mPAP and mDAP was taken to indicate that the distal bypass during the experiment was functioning effectively.
Data analysis
All data were recorded by a monitor (PowerLab; ADInstruments, Castle Hill, Australia), which allowed constant real-time recording of arterial blood pressures (mPAP, mDAP, and SAP (SCPP)) and SCBF. Data were entered into a database and analyzed using SPSS statistical software (version 22.0J; IBM Corp., Armonk, NY). Rates of change in SVR and SCVR were calculated from mSBP, SCPP, and SCBF using the methods described above.
Scatter plots of SVR and SCVR were created, and levels of correlation were analyzed with Spearman’s rank correlation coefficient and regression analysis. Probability values were considered significant at the 5% level.
| Results | ▴Top |
Condition 1: no spinal ischemia (control group)
SCBF increased in accordance with the increase in mSBP induced by noradrenaline administration (Fig. 3a). When values were shown as a scatter plot, a strong positive correlation between SVR and SCVR was evident, and a simple regression analysis yielded an equation that related the two variables with high predictive accuracy, with a coefficient of determination of 0.79 (y = 0.2 + 0.74x, r = 0.889, r2 = 0.789; P < 0.01).
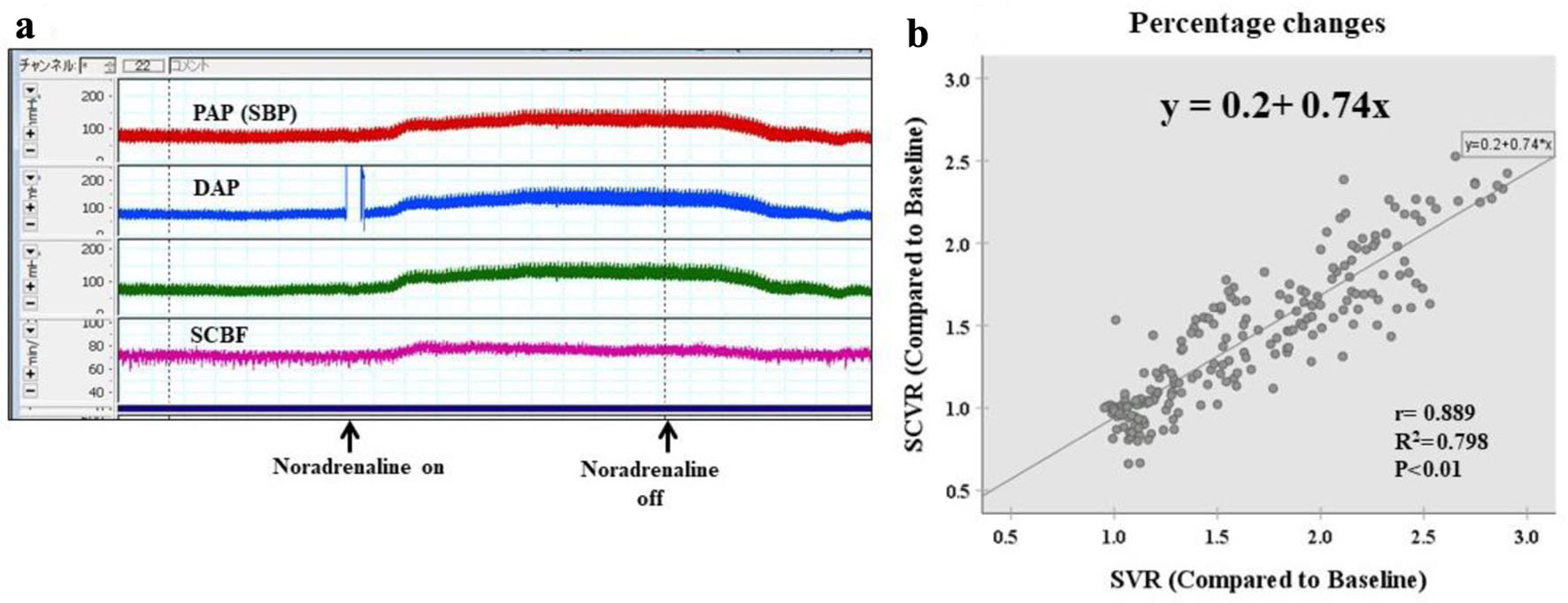 Click for large image | Figure 3. Condition 1: no aortic clamp and no SA clamp (no spinal ischemia: control group). (a) Laboratory chart shows the real-time record of SBP, DAP, and SCBF. SCBF increased in accordance with the increase in SBP induced by noradrenaline administration. (b) Scattergram of percentage changes shows a strong positive correlation between SVR and SCVR, and simple regression analysis yields an equation that relates the two variables with high predictive accuracy, with a coefficient of determination of 0.79 (y = 0.2 + 0.74x, r = 0.889, r2 = 0.789, P < 0.01). DAP: distal aortic blood pressure; PAP: proximal arterial blood pressure; SBP: systemic blood pressure; SCBF: spinal cord blood flow; SCVR: spinal cord vascular resistance; SVR: systematic vascular resistance. |
The x coefficient of 0.74 showed that the rate of change (rate of increase) in SCVR was 0.74 times the rate of change (rate of increase) in SVR, indicating that flow distribution to the spinal cord increased (Fig. 3b).
Condition 2: six pairs of SAs clamped (with distal perfusion)
Under this condition, inflow from the SAs at L2-L7 was halted with distal perfusion. When inflow from the SAs was stopped, SCBF and SAP (SCPP) fell, but rose again as mSBP increased (Fig. 4a). A weak correlation between the rate of change in SVR and the rate of change in SCVR was evident (y = 0.39 + 0.07x, r = 0.209, r2 = 0.039; P < 0.01).
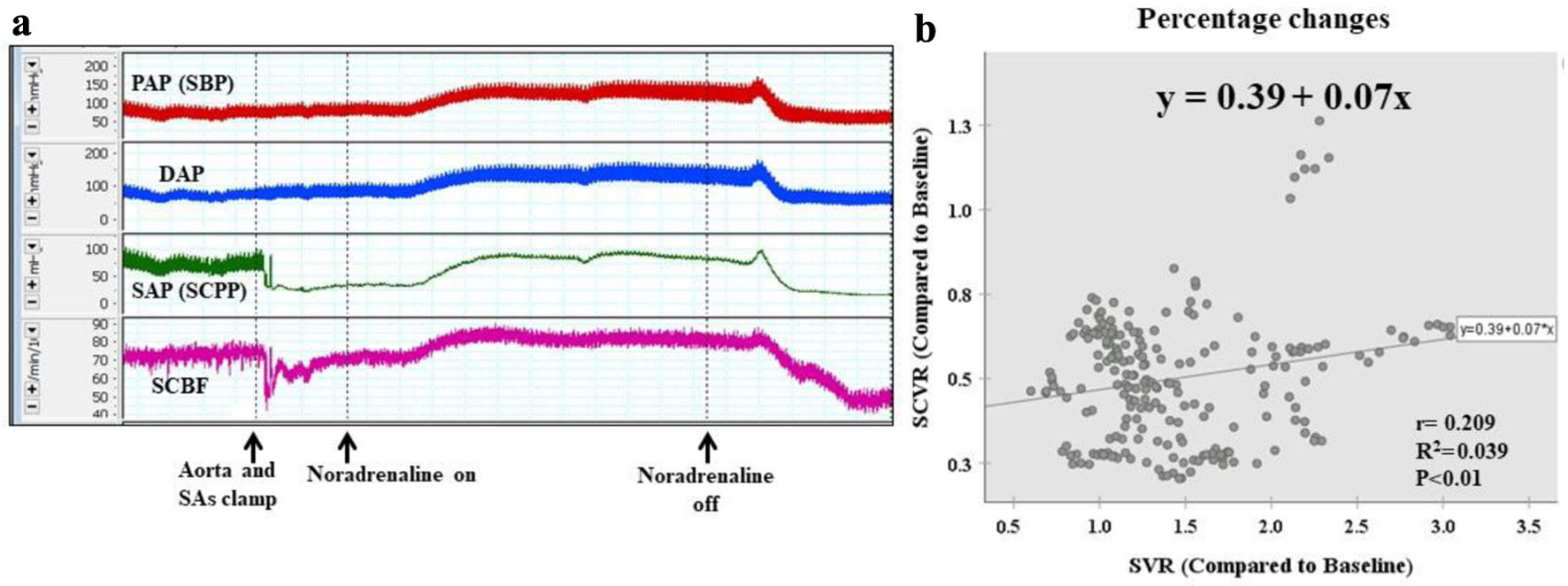 Click for large image | Figure 4. Condition 2: six pairs of SAs clamped (with distal perfusion). (a) When inflow from the SAs is stopped, SCBF and SAP (SCPP) decrease. However, they increase as systemic blood pressure increases. (b) Scattergram of percentage changes shows a weak correlation between the rate of change in SVR and the rate of change in SCVR (y = 0.39 + 0.07x, r = 0.209, r2 = 0.039, P < 0.01). The rate of increase in SCVR is far lower than the rate of increase in SVR. DAP: distal arterial blood pressure; PAP: proximal arterial blood pressure; SA: segmental artery; SAP: segmental arterial pressure; SBP: systemic blood pressure; SCBF: spinal cord blood flow; SCPP: spinal cord perfusion pressure; SCVR: spinal cord vascular resistance; SVR: systematic vascular resistance. |
With SA clamping, the rate of increase in SCVR was far lower than the rate of increase in SVR, indicating that noradrenaline administration had created an environment in which the flow distribution to the spinal cord was likely to increase. However, SCVR did not exceed 80% of the control value, arterioles collapsed, and the blood supply to the spinal cord was in an unstable state (Fig. 4b).
Condition 3: six pairs of SAs clamped (without distal perfusion)
Following condition 2, distal perfusion was halted. When the distal bypass was clamped, SCBF and SAP (SCPP) fell further, but then rose as mSBP increased (Fig. 5a). A weak correlation between the rate of change in SVR and the rate of change in SCVR was evident (y = 0.19 + 0.08x, r = 0.379, r2 = 0.144; P < 0.01).
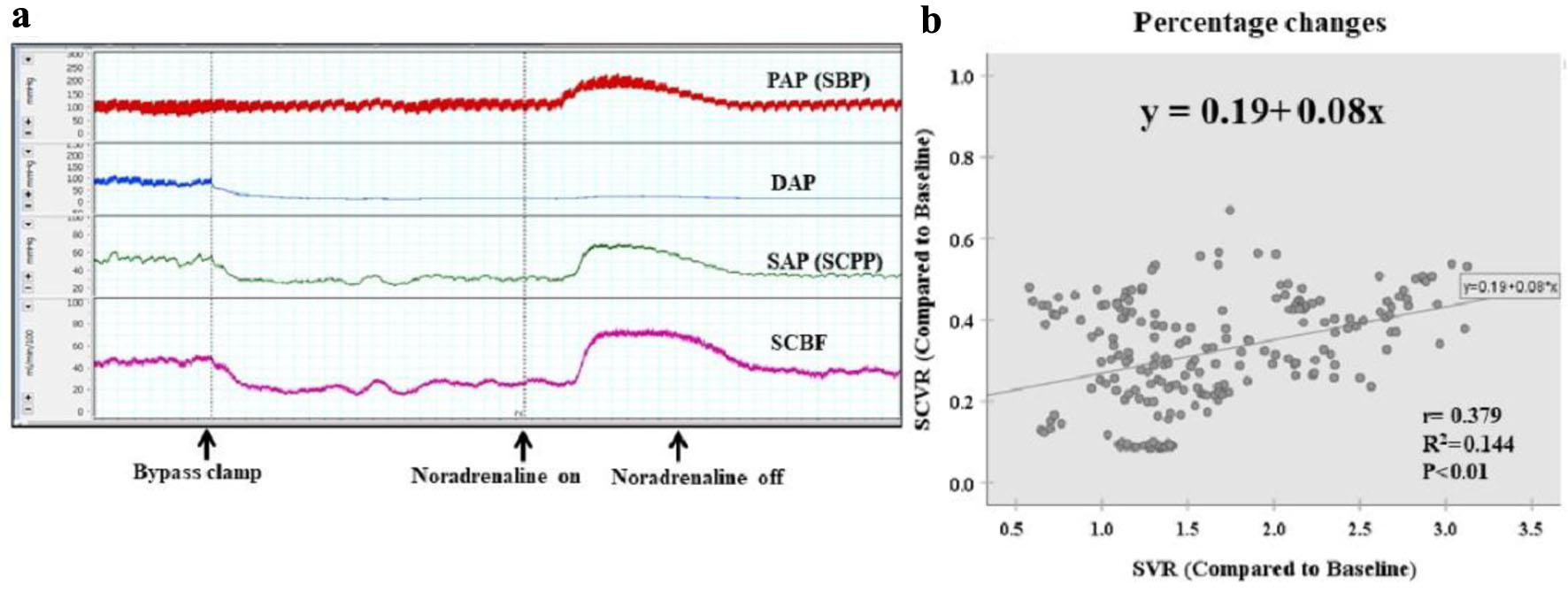 Click for large image | Figure 5. Condition 3: six pairs of SAs clamped (without distal perfusion). (a) When the distal bypass is clamped, SCBF and SAP (SCPP) decrease further. However, they then increase as systemic blood pressure increases. (b) Scattergram of percentage changes shows a weak correlation between the rate of change in SVR and the rate of change in SCVR (y = 0.19 + 0.08x, r = 0.379, r2 = 0.144, P < 0.01). Without distal perfusion, the rate of increase in SCVR is even lower than under condition 2. DAP: distal arterial blood pressure; PAP: proximal arterial blood pressure; SAs: segmental arteries; SAP: segmental arterial pressure; SBP: systemic blood pressure; SCBF: spinal cord blood flow; SCPP: spinal cord perfusion pressure; SCVR: spinal cord vascular resistance; SVR: systematic vascular resistance. |
Without distal perfusion, the rate of increase in SCVR was even lower than that under condition 2. SCVR did not exceed 60% of the control value, arterioles became severely collapsed, and the blood supply was in an extremely unstable state.
| Discussion | ▴Top |
Our results in this study demonstrated that the mechanism by which noradrenaline administration increases the flow to the spinal cord involves the fact that the rate of increase in SCVR is lower than the rate of increase in SVR.
SCII during thoracoabdominal aortic aneurysm surgery requiring extensive SA clamping occurs at a rate of approximately 7-8%, even in highly experienced institutions that perform many such operations. This represents an issue that has yet to be resolved [14, 15]. When antegrade perfusion to the spinal cord is interrupted, the blood supply is heavily dependent on the collateral network via the subclavian artery, intercostal arteries, internal iliac artery, and paraspinal muscles. Various experiments have demonstrated that increasing mSBP offers an effective means of encouraging this collateral blood flow [9, 16, 17]. Numerous clinical studies have shown that maintaining perioperative mSBP at a high level of ≥ 80 - 90 mm Hg represents an important means of perioperative spinal cord protection [3-5, 18].
Although increasing blood pressure by means of β-adrenoreceptor agonists is considered effective, α-adrenoreceptor agonist administration raises concerns that excessive constriction of the spinal resistance vessels may also cause a decrease in SCBF. A previous basic study using a canine model reported that direct administration of an α-adrenoreceptor agonist into the pia mater of the spinal cord caused contraction of the spinal arterioles [19]. As yet, insufficient evidence has been accumulated to determine whether such agents should be selected. In actual clinical practice, however, phenylephrine and noradrenaline are used to increase SCBF when this flow has been decreased by the sacrifice of spinal SAs. When these agents are used to increase mSBP, SCBF from collateral networks increases, and this is reportedly effective in reducing SCII [1, 4].
In neurosurgery and orthopedic surgery, the use of noradrenaline to maintain organ perfusion pressure in the case of traumatic brain or spinal cord injury is widely recommended [6-8]. In previous animal experiments, we have also observed that noradrenaline administration caused both SCBF and SCPP to increase in extensive SA clamping and non-clamping models [9]. In an experimental study by Kurita et al using near-infrared spectroscopy as a method of evaluating spinal cord circulation in order to assess the balance of oxygen supply and demand in the spinal cord, the use of an α-adrenoreceptor agonist was seen to increase O2 delivery to the spinal cord [20]. Noradrenaline has thus been experimentally demonstrated to improve spinal cord circulation from the perspectives of both SCBF and O2 delivery.
The maintenance of postoperative hypertension by noradrenaline may help prevent delayed spinal cord injury. Under conditions of reduced anterograde perfusion to the spinal cord, as in conditions 2 and 3 of this study, the spinal arterioles are dependent on blood supplied via collateral networks and are therefore unable to achieve sufficient vasodilation, resulting in a continued state of collapse. Basic and clinical studies conducted by Etz et al also showed that after extensive spinal cord SA sacrifice, the time required for the SCPP to return to baseline was 72 - 120 h, when the value at 24 h postoperatively was taken as the minimum. Delayed SCII is highly likely to occur during this time [21, 22]. Even in such an ischemic environment, however, spinal cord vascular network remodeling proceeds from day 3 to day 5, resulting in the construction of a stable blood supply network [23, 24]. Remodeling construction at this comparatively early stage (72 - 120 h) is the result of arteriogenesis [24, 25]. This mechanism consists of the opening of collateral channels connecting the interrupted routes of blood flow with those that are still patent, allowing interrupted blood flow routes to supply blood again. The higher the pressure gradient, the easier it is for these channels to open [26]. Maintaining hypertension by postoperative noradrenaline administration generates a high pressure gradient, which encourages remodeling through arteriogenesis, and is considered to be effective in reducing delayed paraplegia.
In this study, the rate of increase in SCVR when noradrenaline was administered under condition 1 (normal condition) was 0.74 times the rate of increase in SVR. Under condition 2, when six pairs of SAs (including the AKA) were clamped, spinal vessels maintained a certain degree of resistance, but this did not exceed 80% of the baseline value, indicating that blood supply from collateral channels did not enable adequate vasodilation. When distal perfusion was interrupted, vascular resistance did not exceed 60% of the baseline, suggesting that the blood supply had collapsed and was in a poor state. This model is based on actual clinical practice, and is useful for understanding the perioperative spinal cord circulation.
Limitations
As with all animal experiments, questions remain regarding whether the conclusions drawn from this study are directly applicable to the clinical management of human beings. However, deepening our understanding of the spinal cord blood supply using large-animal models of spinal cord ischemia remains important [27]. Vascularization of the canine spinal cord is similar to that of human beings [28]. Consequently, a number of studies have used canine models of spinal cord ischemia to evaluate the spinal cord blood supply environment and electrophysiology [29, 30]. The effect of ketamine on SCBF is not well understood, but during thoracoabdominal aortic aneurysm surgery, the amplitude of trans-cranial motor evoked potential may be reduced by the depth of intraoperative anesthesia, making it difficult to assess the spinal cord blood supply environment by electrophysiological evaluation. Therefore, ketamine, which has less influence on Tc-MEP, is preferred for thoracoabdominal aneurysm surgery. When placing the laser Doppler flowmetry probe on the dura mater of the spinal cord, damage to the collateral network caused by cutting the erector spinae muscles and vertebral body dorsal resection was kept to a minimum. In this experiment, we were unable to measure the CSFP accurately due to technical problems. CSFP was therefore assumed to be a uniform 6 mm Hg, and SCPP was defined as SAP - 6 mm Hg. The SCPP indicated in this experiment should be used as an approximation for the actual SCPP. From the pharmacological mechanism of action of noradrenaline, CO remains stable or decreases only slightly [10, 11], and the rate of change in mSBP was therefore used as an approximation for the rate of change in SVR. The use of a Swan-Ganz catheter for CO measurement would enable SVR to be calculated with greater accuracy. This study is significant because we compared changes in SCVR and SVR as a result of noradrenaline administration, and were able to observe blood flow distribution tendencies in closed networks within individual animals. Simply reducing blood flow in the central nervous system does not lead to neurological damage. Normal and pathological conditions exert different effects on the organ when blood flow is impaired. In fact, conditions such as ischemia-reperfusion, blood-brain barrier collapse, and hypothermia must also be taken into account as conditions and events that affect vasoactive agents.
Conclusion
Noradrenaline administration increases SCBF by elevating mSBP due to the fact that the rate of increase in SCVR is lower than the rate of increase in SVR, and the flow distribution to the spinal cord increases as a result. Noradrenaline may potentially be one of the vasopressors that can be recommended for use to avoid SCII.
Acknowledgments
None to declare.
Financial Disclosure
This study was supported by the Japan Society for the Promotion of Science KA_KENHI (grant no. JP21K16498).
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Yuya Kise and Yukio Kuniyoshi conceived the idea of the study. Yuya Kise and Moriyasu Nakaema contributed to the interpretation of the results. Keita Miyaishi, Mizuki Ando, Shotaro Higa and Tatuya Maeda did the literacy search. Hitoshi Inafuku, Moriyasu Nakaema and Kojiro Furukawa supervised the conduct of this study. All authors reviewed the manuscript draft and revised it critically on intellectual content. All authors approved the final version of the manuscript to be published.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
AKA: artery of Adamkiewicz; CO: cardiac output; CSFP: cerebrospinal fluid pressure; DAP: distal arterial blood pressure; ΔP: organ perfusion pressure; mSBP: mean systemic blood pressure; OF: organ flow; OVR: organ vascular resistance; PAP: proximal arterial blood pressure; SAs: segmental arteries; SAP: segmental arterial blood pressure; SCBF: spinal cord blood flow; SCII: spinal cord ischemic injury; SCPP: spinal cord perfusion pressure; SCVR: spinal cord vascular resistance; SVR: systematic vascular resistance
| References | ▴Top |
- Sinha AC, Cheung AT. Spinal cord protection and thoracic aortic surgery. Curr Opin Anaesthesiol. 2010;23(1):95-102.
doi - Ullery BW, Cheung AT, Fairman RM, Jackson BM, Woo EY, Bavaria J, Pochettino A, et al. Risk factors, outcomes, and clinical manifestations of spinal cord ischemia following thoracic endovascular aortic repair. J Vasc Surg. 2011;54(3):677-684.
doi - Etz CD, Weigang E, Hartert M, Lonn L, Mestres CA, Di Bartolomeo R, Bachet JE, et al. Contemporary spinal cord protection during thoracic and thoracoabdominal aortic surgery and endovascular aortic repair: a position paper of the vascular domain of the European Association for Cardio-Thoracic Surgerydagger. Eur J Cardiothorac Surg. 2015;47(6):943-957.
doi - Kemp CM, Feng Z, Aftab M, Reece TB. Preventing spinal cord injury following thoracoabdominal aortic aneurysm repair: The battle to eliminate paraplegia. JTCVS Tech. 2021;8:11-15.
doi pubmed pmc - Miller LK, Patel VI, Wagener G. Spinal Cord Protection for Thoracoabdominal Aortic Surgery. J Cardiothorac Vasc Anesth. 2022;36(2):577-586.
doi - Altaf F, Griesdale DE, Belanger L, Ritchie L, Markez J, Ailon T, Boyd MC, et al. The differential effects of norepinephrine and dopamine on cerebrospinal fluid pressure and spinal cord perfusion pressure after acute human spinal cord injury. Spinal Cord. 2017;55(1):33-38.
doi - Muzevich KM, Voils SA. Role of vasopressor administration in patients with acute neurologic injury. Neurocrit Care. 2009;11(1):112-119.
doi - Menacho ST, Floyd C. Current practices and goals for mean arterial pressure and spinal cord perfusion pressure in acute traumatic spinal cord injury: Defining the gaps in knowledge. J Spinal Cord Med. 2021;44(3):350-356.
doi pubmed pmc - Kise Y, Kuniyoshi Y, Inafuku H, Nagano T, Hirayasu T, Yamashiro S. Directly measuring spinal cord blood flow and spinal cord perfusion pressure via the collateral network: correlations with changes in systemic blood pressure. J Thorac Cardiovasc Surg. 2015;149(1):360-366.
doi - Brunton LL, Chabner B, Goodman LS, Knollmann BC. Goodman & Gilman’s the pharmacological basis of therapeutics, 12th Edition. New York, N.Y.: McGraw-Hill Education LLC; 2011.
- Goldenberg M, Apgar V, et al. Nor-epinephrine (arterenol, sympathin N) as a pressor drug. J Am Med Assoc. 1949;140(9):776-778.
doi - Endo K, Hara Y, Nezu Y, Tgawa M. Measurement of cerebrospinal fluid pressure of dogs by use of the fiber optic catheter. Japanese Journal of Veterinary Anesthesia & Surgery. 2008;39(2):29-33.
- Doppman JL, Ramsey R. Selective arteriography of the lumbar spinal cord in dogs. Neuroradiology. 1971;3(2):64-67.
doi - Moulakakis KG, Karaolanis G, Antonopoulos CN, Kakisis J, Klonaris C, Preventza O, Coselli JS, et al. Open repair of thoracoabdominal aortic aneurysms in experienced centers. J Vasc Surg. 2018;68(2):634-645.e612.
doi - Rocha RV, Lindsay TF, Friedrich JO, Shan S, Sinha S, Yanagawa B, Al-Omran M, et al. Systematic review of contemporary outcomes of endovascular and open thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2020;71(4):1396-1412.e1312.
doi - Taira Y, Marsala M. Effect of proximal arterial perfusion pressure on function, spinal cord blood flow, and histopathologic changes after increasing intervals of aortic occlusion in the rat. Stroke. 1996;27(10):1850-1858.
doi - Izumi S, Okada K, Hasegawa T, Omura A, Munakata H, Matsumori M, Okita Y. Augmentation of systemic blood pressure during spinal cord ischemia to prevent postoperative paraplegia after aortic surgery in a rabbit model. J Thorac Cardiovasc Surg. 2010;139(5):1261-1268.
doi - Tanaka A, Estrera AL, Safi HJ. Open thoracoabdominal aortic aneurysm surgery technique: how we do it. J Cardiovasc Surg (Torino). 2021;62(4):295-301.
doi - Iida H, Ohata H, Iida M, Watanabe Y, Dohi S. Direct effects of alpha1- and alpha2-adrenergic agonists on spinal and cerebral pial vessels in dogs. Anesthesiology. 1999;91(2):479-485.
doi - Kurita T, Kawashima S, Morita K, Nakajima Y. Spinal cord autoregulation using near-infrared spectroscopy under normal, hypovolemic, and post-fluid resuscitation conditions in a swine model: a comparison with cerebral autoregulation. J Intensive Care. 2020;8:27.
doi pubmed pmc - Etz CD, Homann TM, Plestis KA, Zhang N, Luehr M, Weisz DJ, Kleinman G, et al. Spinal cord perfusion after extensive segmental artery sacrifice: can paraplegia be prevented? Eur J Cardiothorac Surg. 2007;31(4):643-648.
doi - Etz CD, Zoli S, Bischoff MS, Bodian C, Di Luozzo G, Griepp RB. Measuring the collateral network pressure to minimize paraplegia risk in thoracoabdominal aneurysm resection. J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S125-S130; discussion S142-S146.
doi - Geisbusch S, Schray D, Bischoff MS, Lin HM, Griepp RB, Di Luozzo G. Imaging of vascular remodeling after simulated thoracoabdominal aneurysm repair. J Thorac Cardiovasc Surg. 2012;144(6):1471-1478.
doi pubmed pmc - Etz CD, Kari FA, Mueller CS, Brenner RM, Lin HM, Griepp RB. The collateral network concept: remodeling of the arterial collateral network after experimental segmental artery sacrifice. J Thorac Cardiovasc Surg. 2011;141(4):1029-1036.
doi pubmed pmc - Simon F, Wagenhauser MU, Busch A, Schelzig H, Gombert A. Arteriogenesis of the Spinal Cord-The Network Challenge. Cells. 2020;9(2):501.
doi pubmed pmc - Simons M. Angiogenesis: where do we stand now? Circulation. 2005;111(12):1556-1566.
doi - Mazensky D, Flesarova S, Sulla I. Arterial Blood Supply to the Spinal Cord in Animal Models of Spinal Cord Injury. A Review. Anat Rec (Hoboken). 2017;300(12):2091-2106.
doi - Fletcher TF, Kitchell RL. Anatomical studies on the spinal cord segments of the dog. Am J Vet Res. 1966;27(121):1759-1767
- Kato S, Kawahara N, Tomita K, Murakami H, Demura S, Fujimaki Y. Effects on spinal cord blood flow and neurologic function secondary to interruption of bilateral segmental arteries which supply the artery of Adamkiewicz: an experimental study using a dog model. Spine (Phila Pa 1976). 2008;33(14):1533-1541.
doi - Fujimaki Y, Kawahara N, Tomita K, Murakami H, Ueda Y. How many ligations of bilateral segmental arteries cause ischemic spinal cord dysfunction? An experimental study using a dog model. Spine (Phila Pa 1976). 2006;31(21):E781-789.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


