| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 14, Number 5, October 2023, pages 360-369
Long-Term Assessment of Thin-Strut BioMime Coronary Stent System in Real-World Population at Single-Center: A Retrospective Observational Study
Girish Meennahalli Palledaa, b, Mohit Guptaa, b, c, Ankit Bansala, Vishal Batraa, Sanjay Tyagia, Shekhar Kunala
aDepartment of Cardiology, Gobind Ballabh Pant Hospital, New Delhi, India
bThese authors contributed equally to this work.
cCorresponding Author: Mohit Gupta, Department of Cardiology, Gobind Ballabh Pant Hospital, New Delhi, India
Manuscript submitted April 22, 2023, accepted September 13, 2023, published online October 21, 2023
Short title: Four-Year Outcomes of an Observational Study
doi: https://doi.org/10.14740/cr1515
| Abstract | ▴Top |
Background: The short-term clinical outcomes of first-generation thicker-strut durable polymer-based drug-eluting stents (DES) have been widely examined. However, there is a scarcity on qualitative research on the long-term usage of DES that evaluated the thinner strut biodegradable stents for coronary artery disease. Hence, we sought to investigate the long-term safety and performance of thinner strut biodegradable polymer-based BioMime sirolimus-eluting coronary stent system in real-world patients with symptomatic ischemic heart disease.
Methods: This was a retrospective, observational, single-center, post-marketing clinical follow-up study. The primary endpoints were the incidence of major adverse cardiac events (MACE), defined as a composite of cardiac death, myocardial infarction (MI) attributed to target vessel revascularization (TVR), and target lesion revascularization (TLR) at 1-, 2-, 3- and 4-year follow-ups. The secondary endpoints were cardiac death, MI, TLR, TVR, device and procedural success rates, and stent thrombosis (ST).
Results: In all, 1,188 consecutive patients were enrolled, and 1,333 (1,257 de novo and 76 in-stent restenotic lesions) out of 1,565 lesions were treated with the study device. The mean age of patients was 53.26 ± 10.31 years and 86.2% were male. The quantitative coronary angiographic derived mean lesion length and diameter were 29.62 ± 9.62 mm and 3.01 ± 0.29 mm, respectively. The average length and diameter of the study device implanted were 30.89 ± 6.31 mm and 3.17 ± 0.25 mm, respectively. The cumulative incidence of MACE at 1-, 2-, 3-, and 4 years was 0.61%, 1.47%, 2.08%, and 3.40%, respectively, and cumulative deaths due to cardiac causes were 0.61%, 1.13%, 1.22%, and 1.83%, respectively. There were no cases of TLR or TVR at 1-year follow-up. The cumulative rate of TLR at 2-, 3-, and 4 years was 0.35%, 0.87%, and 1.57%, respectively, while that of TVR was 0.61%, 1.47%, and 2.35%, respectively. Three (0.3%) incidences of probable ST occurred during the 6-month follow-up; no new cases were reported further. In subgroup analysis, MACEs were comparable across the long- and short-length stent groups through 4-year follow-up.
Conclusions: This long-term study demonstrates the safety and performance of the ultra-thin BioMime sirolimus-eluting stent with satisfactory clinical outcomes in patients with symptomatic ischemic heart disease in real-world scenario.
Keywords: Biodegradable polymers; BioMime stent; Coronary artery disease; Long-term; Major adverse cardiovascular events; Percutaneous coronary intervention; Stent thrombosis; Thinner-strut
| Introduction | ▴Top |
Percutaneous coronary intervention (PCI) is an increasingly performed revascularization modality for the treatment of coronary artery disease (CAD) [1]. PCI with drug-eluting stent (DES) implantation has become a standard therapeutic procedure for patients with CAD [2]. The use of DES has been reported to be more effective than bare metal stents in the prevention of angiographic and clinical restenosis [2]. The radial strength of DES and improved deployment techniques facilitate optimal stent deployment in complex lesions [3]. This has further reduced the incidence of restenosis and repeat revascularization in single-vessel de novo disease [4].
Nevertheless, concerns have been raised regarding the potential increased risk of late and very late stent thrombosis (ST) following DES implantation [5]. This is perceived to be a consequence of delayed endothelialization [6]. The newer-generation DES have thinner struts and biocompatible polymers, which reduce vessel wall injury, decrease inflammation, and promote faster endothelialization [7, 8]. However, with increased usage of DES in complex lesions (e.g., bifurcations, lesions requiring overlapping stents, acute myocardial infarctions (MI)), and in patients with clinical conditions like diabetes and chronic kidney disease, there is a small yet substantial risk of ST and in-stent restenosis. Thrombosis can occur years after implantation and premature discontinuation of antiplatelet therapy is an additional risk factor for ST. Hence, long-term evaluation of the outcomes with biodegradable polymer-based newer DES is necessary in real-world settings.
The BioMime sirolimus-eluting stent (SES) system (Meril Life Sciences Pvt. Ltd, India) is a Conformite Europeenne (CE)-marked new-generation DES system, which incorporates an advanced ultra-thin strut platform (65-µm) covered with a biodegradable polymer to release sirolimus, an antiproliferative drug, in the vessel wall. The clinical safety and efficacy of this stent were demonstrated in meriT-1 [9], meriT-2 [10], meriT-3 [11], and meriT-V [12] trials. However, in all these trials, the outcomes were assessed for only up to 12 months. The long-term performance of this stent is still not evaluated. In this retrospective study, we aimed to evaluate the incidence rates of major adverse cardiac events (MACE), and ST associated with the biodegradable polymer BioMime SES at the end of 4 years, in real-world settings. The primary and secondary endpoints of the study were successfully met.
| Materials and Methods | ▴Top |
Study design and population
This retrospective, single-center, post-marketing clinical follow-up study was conducted to evaluate the safety and performance of the BioMime SES in patients with CAD in real-world settings over 4 years. All consecutive patients with CAD who were treated with BioMime SES in a large tertiary care center from December 2015 to December 2018 were enrolled in the study. The study included 1,188 all-comer patients above 18 years of age who were implanted with at least one BioMime SES. BioMime SES system is indicated for improving coronary luminal diameter in patients with symptomatic ischemic heart disease due to de novo and in-stent restenotic lesions (lengths ≤ 44 mm) in native coronary arteries with a reference vessel diameter of 2.00 to 4.5 mm in patients eligible for percutaneous transluminal coronary angioplasty and stenting procedures. Patients who were part of any other investigational study were excluded. The Institutional Ethics Committee approved the protocol (approval number: F.1/IEC/MAMC/185/03/2021/No.419) and exempted the requirement for written informed consent without any alteration in the management of patients.
Coronary interventional procedures were performed per the standard guidelines [13]. Dual antiplatelet therapy (DAPT), including a loading dose of aspirin (300 mg) and clopidogrel (600 mg) or prasugrel (60 mg) or ticagrelor (180 mg), was given to each patient. Procedural anticoagulation was achieved either with heparin or bivalirudin. However, intra-procedural GPIIb/IIIa inhibitor administration was at the investigator’s discretion. All the patients were advised to maintain DAPT (aspirin 75 - 300 mg daily indefinitely and clopidogrel 75 mg daily or prasugrel 10 mg daily or ticagrelor 90 mg twice daily for at least 12 months) after the procedure.
Ethical compliance statement
This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Device description and procedural details
Earlier publications have provided in-depth descriptions of the technical details and distinctive features of the BioMime SES [9-12]. BioMime SES is a novel balloon-expandable system with a hybrid architecture (closed and open cells) that facilitates morphology-mediated expansion and has an ultra-thin (65 µm) strut. It is coated with biodegradable polymers poly lactic-co-glycolic acid (PLGA) and poly-L-lactic acid (PLLA) and developed on cobalt-chromium alloy platform, with sirolimus as an antiproliferative agent. The device is commercially available in varied lengths and diameters. The PCI procedures were performed in accordance with the standard American Heart Association guidelines [14].
Definitions and endpoints
The primary endpoint was MACE, defined as a composite of cardiac death, MI attributed to target vessel revascularization (TVR), and target lesion revascularization (TLR) up to 4 years of follow-up. Secondary endpoints were cardiac death, MI, TVR, and TLR. The ST was classified and defined according to the Academic Research Consortium (ARC) [13]. Definite ST was confirmed either angiographically or pathologically: probable ST: any unexplained death within the first 30 days after intracoronary stenting; possible ST: occurred with any unexplained death from 30 days after intracoronary stenting until end of the follow-up. Procedural success was defined as the successful delivery and deployment of the study stent at the desired position with < 30% residual stenosis by quantitative coronary angiography. Device success was defined as procedural success without the occurrence of cardiac death related to the target vessel, target vessel myocardial infarction (TVMI), or repeat TLR during the hospital stay.
Follow-up
The clinical data were reviewed and collected at 1-, 2-, 3-, and 4 years as available in hospital medical records and also by telephonic follow-up. Data were collected relating to current clinical status, any hospitalization, and the occurrence of any MACE.
Data collection
All baseline characteristics, demographics, current medications, procedural details, discharge summaries, and outcome data were collected from hospital records and retrospectively extrapolated for the current analysis. The primary and secondary endpoint related (any adverse/serious adverse event) data as per their availability through hospital medical records and by telephonic follow-up from patients, were documented using paper case report forms.
Statistical analysis
A single-arm design was used to test whether the proportion is different from 0.098 (assumed MACE rate of 9.8% from the literature) [15]. The comparison was made using a two-sided, one-sample exact test, with a type I error rate (α) of 0.05. To detect a difference of 0.033 with 91.4% power, this study design required 1,000 subjects.
The demographic and baseline characteristics are summarized using descriptive statistics. Continuous variables like age are represented as mean ± standard deviation (SD) while other categorical variables such as gender, cardiac status, and risk factors are reported as frequencies and percentages. Percentage calculation involved only the number of patients whose data were available. A subgroup analysis was performed to compare the BioMime SES groups who received the long and short stents in terms of pertinent adverse events. Statistical analysis was performed using a statistical package for social sciences (SPSS v23.0, IBM Corp., Somers, NY, USA).
| Results | ▴Top |
Baseline and demographic characteristics
A total of 1,188 patients were enrolled in the study. The mean age was 53.26 ± 10.31 years, and 86.2% of patients were male. In the total cohort, 650 (54.71%) patients had a history of smoking, and comorbidities such as hypertension and diabetes mellitus were present in 273 (22.98%) and 202 (17%) patients, respectively. At baseline, stable angina, unstable angina, ST-elevation MI and non-ST-elevation MI were present in 1,007 (84.76%), 37 (3.11%), 134 (11.28%) and 10 (0.84%) patients, respectively. The baseline demographics and clinical characteristics of patients are shown in Table 1.
 Click to view | Table 1. Baseline Demographics and Clinical Characteristics |
Lesion and procedural characteristics
In 1,188 patients, there were a total of 1,565 lesions. Out of these, 1,333 lesions (1,257 de novo lesions and 76 in-stent stenosis) were treated with 1,344 BioMime SES (1.13 stents per patient). Chronic total occlusion was seen in 327 (25.31%) patients, while bifurcation lesions were found in 106 (7.9%) patients. A total of 675 (56.82%) patients had single-vessel disease, 130 (10.94%) had double-vessel disease and 383 (32.24%) had multiple vessel disease. The most common target vessel was the left anterior descending artery in 514 (40.89%) patients followed by right coronary artery in 500 (39.78%) and left circumflex artery in 232 (18.46%) patients. Lesions were also seen in the proximal (n = 488; 36.6%) and mid (n = 594; 44.56%) sections of the coronary arteries. The mean lesion length and diameter was 29.62 ± 9.62 mm and 3.01 ± 0.29 mm, respectively. The average length and diameter of the study device was 30.89 ± 6.31 mm and 3.17 ± 0.25 mm, respectively. In all, 232 lesions were treated with 240 stents other than the study device. The mean length and diameter of other stents was 26.83 ± 9.23 mm and 2.89 ± 0.61 mm, respectively (Table 2). The procedural as well as device success was achieved in 100% of the patients with no MACE or any adverse event during the index procedure. The majority of the patients were prescribed clopidogrel (61.03%), followed by ticagrelor (26.01%) and prasugrel (12.96%) (Table 2).
 Click to view | Table 2. Lesion and Procedural Characteristics |
Clinical outcomes through 1-year to 4-year follow-up
In all, 1,154 and 1,129 patients completed the 1- and 2-year follow-up, while 1,107 and 1,100 patients completed the 3- and 4-year follow-up, respectively. Figure 1 shows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) flow diagram of patients (non-cumulative) who were enrolled and followed up for 4 years. The cumulative incidence of MACE at 1-, 2-, 3-, and 4 years was 7 (0.61%), 17 (1.47%), 24 (2.08%), and 39 (3.4%), respectively. The cumulative deaths due to cardiac causes at 1-, 2-, 3-, and 4 years were seven (0.61%), 13 (1.13%), 14 (1.22%), and 21 (1.83%), respectively. There was no case of TLR or TVR at 1-year follow-up. The cumulative rate of TLR at 2, 3, and 4 years was four (0.35%), 10 (0.87%), and 18 (1.57%), respectively, while that of TVR was seven (0.61%), 17 (1.47%), and 27 (2.35%), respectively. There were three (0.3%) incidences of probable ST as per ARC definitions during the 6-month follow-up and no new cases were reported during the subsequent follow-up period (Table 3).
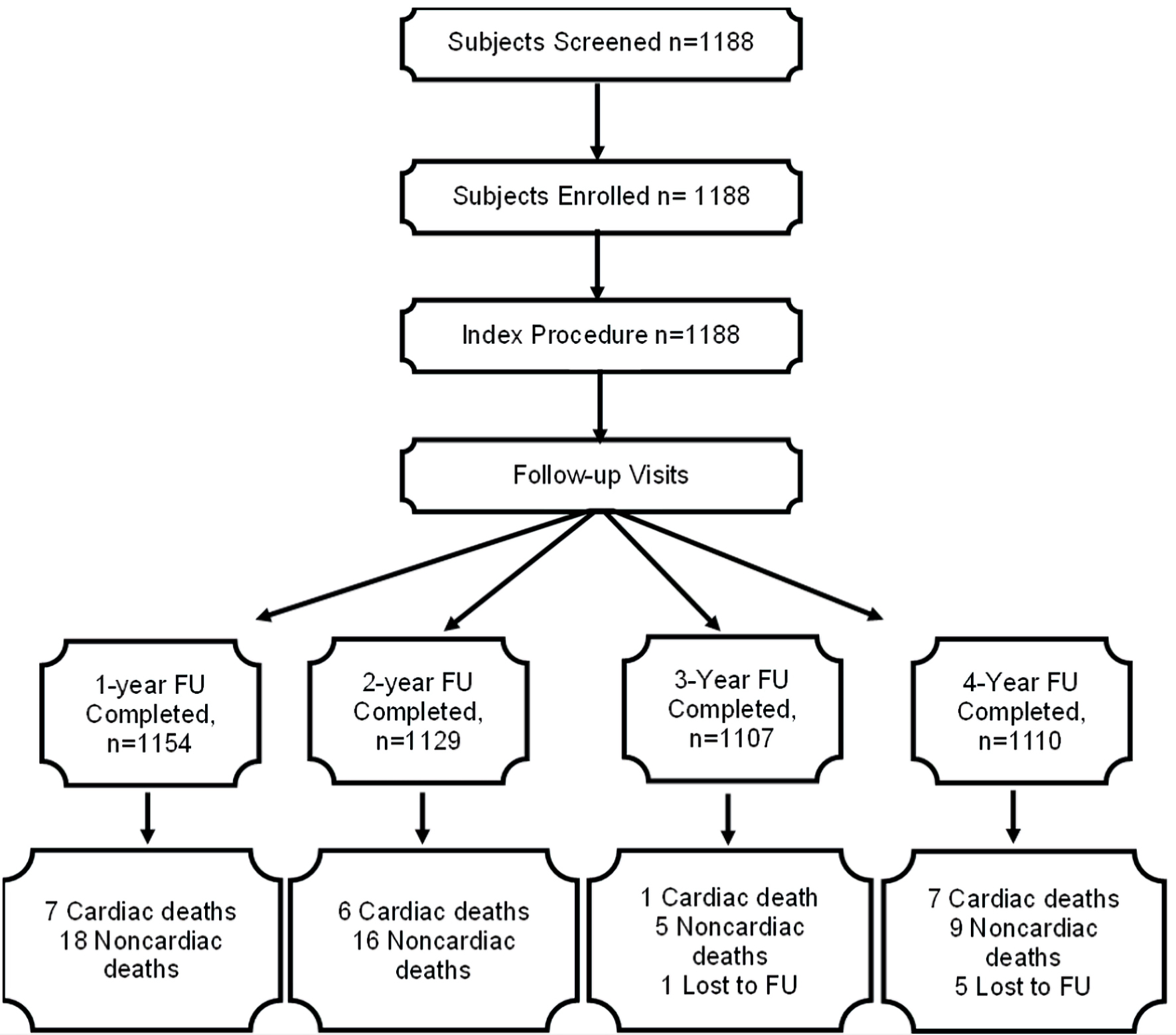 Click for large image | Figure 1. The STROBE flow diagram of patients (non-cumulative) who were enrolled and followed-up for 4 years. STROBE: Strengthening the Reporting of Observational Studies in Epidemiology; FU: follow-up. |
 Click to view | Table 3. Cumulative Major Adverse Cardiac Events at 1-Year to 4-Year Follow-Up |
Subgroup analysis
A subgroup analysis was carried out to compare the rate of MACE between the long stent (≥ 35 mm) and short stent length (< 35 mm) groups. It was revealed that the rates of MACE were comparable between the two groups (Table 4).
 Click to view | Table 4. Comparison of Cumulative Major Adverse Cardiac Events Between Long Stent (≥ 35 mm) and Short Stent (< 35 mm) Groups |
| Discussion | ▴Top |
In this retrospective study in “real-world” settings among patients with CAD, the use of the BioMime SES system has demonstrated satisfactory safety and performance outcomes including device and procedural success. The cumulative rate of MACE at 4 years was 3.40%, while that of TLR and TVR was 1.57% and 2.35%, respectively. There were three (0.3%) incidences of probable ST during the 6-month follow-up, and no new cases were reported during the subsequent follow-up period. The rate of cardiac death was 1.83% (n = 21) at 4-year follow-up. MACE events were comparable between long-length and short-length BioMime SES groups through 4-year follow-up.
Previous studies have reported outcomes with BioMime SES over shorter periods of follow-up. The meriT-1 trial, which was the initial human evaluation of the BioMime SES in patients with single de novo coronary lesions reported a 0% rate of MACE and ST at 12 months [9]. This was lower than the rate of MACE and ST at 12 months in the current study, which was 0.61% and 0.3%, respectively. However, the meriT-1 trial included only 30 patients. In contrast, our study had 1,188 patients and included populations with multiple as well as non-de novo lesions. Moreover, stents other than BioMime SES were used in approximately 15% of lesions in our study. The meriT-2 clinical trial among 250 patients in real-world settings reported a 12-month cumulative MACE rate of 6%, including 0.8% cardiac death and 5.2% of TLR (4.8% clinically indicated TLR) and a ST rate of 0.4% [10]. The rates of MACE and TLR at 12 months were considerably higher than those in our study. This could be attributed to the fact that in our study sample size, patients with diabetes and those with a history of prior MI were substantially fewer. The rate of cardiac death in meitT-2 study was 0.8%, which is comparable with the rate of 0.61% seen in our study. In the meriT-3 study involving 1,161 patients, which also had a 1-year follow-up, the rate of MACE was 2.35%, all-cause death was 1.39%, TVR was 0.09%, TLR was 0.52%, and ST was 0.09% [11]. Though the rates of all-cause death and ST were comparable to those of our study, the rates of MACE, TVR, and TLR were higher in the meriT-3 study. However, the prevalence of baseline hypertension and diabetes in the meriT-3 study was almost twice as high as in our study. This might have caused differences in the outcomes. In the recently published meriT-V prospective trial, the incidence of MACE was 2.98%, TVR 0%, TLR 2.98%, and ST 0% at 9-month [12] follow-up. The incidence of baseline hypertension and dyslipidemia in meriT-V trial was quite high (almost 70-75%), which might have led to higher rates of MACE and TLR. Recently, a retrospective analysis utilizing the same device at a single-center reported a 1-year MACE rate of 0.93%, and TLR and ST 0%, which is comparable to the outcomes of the present study [16]. Nevertheless, none of the previous studies with BioMime have reported outcomes beyond 1-year. As a result, we believe that the outcomes of our study are significant with respect to the long-term follow-up.
Several studies have evaluated long-term outcomes with second-generation SES. One of the studies evaluated the outcomes with a SES made of biodegradable base [17]. At 3.6 ± 0.6 years of follow-up, the incidence of MACE and TLR was 3.6% each, and that of cardiac death, TVR, and ST was 0%. These incidences were lower than those observed in our study. However, this study had a much smaller sample size (n = 127) and the proportion of patients who had ACS was lower (7.9% vs. 14.9%). Iniguez et al reported 5-year outcomes using a bioresorbable polymer SES (80 µm) on a cobalt-chromium platform in patients with multivessel CAD. The rates of target lesion failure were 5.3% and 10.2%, cardiac death rates were 2.2% and 4%, TLR rates were 2.2% and 6.2%, ST rates were 0.4% and 0.9%, and TVR rates were 6.2% and 13.3%, respectively at 1-year and 5-year follow-up [18]. The outcomes of the present study are comparable to these findings. Although the proportion of patients with diabetes in their cohort was two-fold higher, the higher strut thickness may have contributed to the less favorable outcomes compared to those seen in our study. Another study (FOCUS registry) involving 4,720 patients who were implanted with a cobalt-chromium SES showed the rate of 3-year MACE of 7.37%, cardiac death 3.52%, TVR 2.08%, and ST 0.72% [19]. The comparison of outcomes of current registry with other contemporary devices is summarized in Table 5 [19-27]. In comparison to other contemporary devices, BioMime SES showed lower rates of cardiac death and MI [20-28]. The rates of TLR are also lower with BioMime SES (0%) when compared with FLEX registry (0.7%) [20], Tetriflex SES (1.72%) [21], T-FLEX registry (1.9%) [22], Genoss DES prospective registry (0.5%) [25], BIOFLOW-VII registry (0.9%) [26], e-Cobra study (4.3%) [27] and LEADERS FREE III study (4.3%) [28], while the TLR rates are comparable with Thailand Orsiro registry (0%) [23] and FlexyRap® DES study (0%) [24]. Comparable ST rates are observed with FlexyRap® DES study [24] and BIOFLOW-VII registry [26], while lower rate of ST is observed when compared with other contemporary device studies [20-23, 25, 27, 28]. BioMime SES also showed lower MACE rates (0.61%) [20-23, 25-28] while the rates are comparable with FlexyRap® DES study (0.4%) [24].
 Click to view | Table 5. Comparison of Clinical Outcomes With Contemporary Devices |
The BioMime SES has a cobalt-chromium base with an ultra-thin stent platform (65 µm) [9]. Strut thickness is a major determinant of local inflammation [10]. Cobalt-chromium metal alloys allow for thinner stent struts, which facilitates crossability without compromising radial strength, fatigue resistance, or radiopacity [29]. Two studies (ISAR-STEREO and ISAR-STEREO-2) confirmed that strut thickness affected the rate of restenosis [30, 31]. Thicker struts lead to delayed full neointimal coverage, which increases the risk of subacute thrombosis. Additionally, thinner struts facilitate improved endothelialization and they are more flexible, which enhances their trackability and crossability [32]. Moreover, thin-strut stent platforms enable the production of extremely low-profile stents, which enhances their flexibility and deliverability [33]. This could have contributed to better long-term follow-up results.
Further, the BioMime SES has a coating of biodegradable polymers like poly lactic-co-glycolic acid (PLGA) and poly-L-lactic acid (PLLA). These polymers disintegrate after the drug has been released, leaving an inert metal stent in place. This may promote arterial healing by eliminating a chronic source of inflammation in the lumen area. Thus, biodegradable polymers improve the safety and efficiency of DES due to their controlled anti-restenotic drug release and coating degradation over time [16]. Also, there are reports demonstrating the improved outcomes of biodegradable DES during the longer follow-up over durable DES [34, 35]. In light of the aforementioned findings, the data of our study are encouraging and favorable, providing valuable evidence of the long-term clinical performance of BioMime SES.
Limitations
The following are some of the limitations of this study: 1) Retrospective and observational nature; 2) Non-randomized, single-arm design necessitates head-to-head comparison with other contemporary DES; 3) Longer-term cardiac imaging data would be required to evaluate the factual event rates and to ascertain the durability of the biodegradable polymer-based, cobalt-chromium BioMime SES.
Conclusions
The sirolimus-eluting novel BioMime coronary stents with ultra-thin struts and a biodegradable polymer base demonstrated a high procedural success rate with low rates of MACE, ST, TVR, and TLR over the long-term period in real-world settings. This validates the prolonged safety and performance of the BioMime SES in a relatively large heterogenous patient population with a high prevalence of comorbidities.
Acknowledgments
We thank Dr. Shailaja Mahajan-Thakur for extending her assistance for the manuscript writing.
Financial Disclosure
No fund, grant, or other support was received.
Conflict of Interest
The authors have no conflict of interest to disclose.
Informed Consent
Informed consent was obtained from all participants included in the study.
Author Contributions
GMP and MG conceptualized and planned the study particulars. AB monitored the progress of the study. VB acquired the data. ST contributed to the analysis and interpretation of data. SK wrote the first draft. All of the authors reviewed the manuscript for significant intellectual content and consented for it to be published.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ACS: acute coronary syndrome; ARC: Academic Research Consortium; CAD: coronary artery disease; DES: drug-eluting stents; MACE: major adverse cardiovascular event; MI: myocardial infarction; PCI: percutaneous coronary intervention; PLGA: poly lactic-co-glycolic acid; PLLA: poly-L-lactic acid; SES: sirolimus-eluting stent system; ST: stent thrombosis; TLR: target lesion revascularization; TVMI: target vessel myocardial infarction; TVR: target vessel revascularization
| References | ▴Top |
- Trimukhe R, Vani P, Patel A, Salgotra V. Safety and performance of the EverPro(TM) everolimus-eluting coronary stent system with biodegradable polymer in a real-world scenario. World J Cardiol. 2020;12(12):615-625.
doi pubmed pmc - Kirtane AJ, Gupta A, Iyengar S, Moses JW, Leon MB, Applegate R, Brodie B, et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119(25):3198-3206.
doi pubmed - Stefanini GG, Holmes DR, Jr. Drug-eluting coronary-artery stents. N Engl J Med. 2013;368(3):254-265.
doi pubmed - Gregg S. Drug-eluting stents - current and future perspectives. Interv Cardiol. 2006;1(1):28-29.
- Farb A, Boam AB. Stent thrombosis redux—the FDA perspective. N Engl J Med. 2007;356(10):984-987.
doi pubmed - Buchanan GL, Basavarajaiah S, Chieffo A. Stent thrombosis: incidence, predictors and new technologies. Thrombosis. 2012;2012:956962.
doi pubmed pmc - Kang SH, Park KW, Kang DY, Lim WH, Park KT, Han JK, Kang HJ, et al. Biodegradable-polymer drug-eluting stents vs. bare metal stents vs. durable-polymer drug-eluting stents: a systematic review and Bayesian approach network meta-analysis. Eur Heart J. 2014;35(17):1147-1158.
doi pubmed - Mahmoud AN, Shah NH, Elgendy IY, Agarwal N, Elgendy AY, Mentias A, Barakat AF, et al. Safety and efficacy of second-generation drug-eluting stents compared with bare-metal stents: An updated meta-analysis and regression of 9 randomized clinical trials. Clin Cardiol. 2018;41(1):151-158.
doi pubmed pmc - Dani S, Costa RA, Joshi H, Shah J, Pandya R, Virmani R, Sheiban I, et al. First-in-human evaluation of the novel BioMime sirolimus-eluting coronary stent with bioabsorbable polymer for the treatment of single de novo lesions located in native coronary vessels - results from the meriT-1 trial. EuroIntervention. 2013;9(4):493-500.
doi pubmed - Seth A, Wander GS, Mullasari A, Nanjappa MC, Heggunje-Shetty P, Alexander T, Hardas S, et al. Late angiographic and clinical outcomes of the novel BioMime™ sirolimus-eluting coronary stent with ultra-thin cobalt-chromium platform and biodegradable polymer for the treatment of diseased coronary vessels: results from the prospective, multicentre meriT-2 clinical trial. Asia Interv. 2016;2:19-27.
- Jain RK, Chakravarthi P, Shetty R, Ramchandra P, Polavarapu RS, Wander GS, Mohan B, et al. One-year outcomes of a BioMime Sirolimus-Eluting Coronary Stent System with a biodegradable polymer in all-comers coronary artery disease patients: The meriT-3 study. Indian Heart J. 2016;68(5):599-603.
doi pubmed pmc - Abizaid A, Kedev S, Kedhi E, Talwar S, Erglis A, Hlinomaz O, Masotti M, et al. Randomised comparison of a biodegradable polymer ultra-thin sirolimus-eluting stent versus a durable polymer everolimus-eluting stent in patients with de novo native coronary artery lesions: the meriT-V trial. EuroIntervention. 2018;14(11):e1207-e1214.
doi pubmed - Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344-2351.
doi pubmed - Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr., Ganiats TG, Holmes DR, Jr., Jaffe AS, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(25):e344-426.
doi pubmed - Kandzari DE, Koolen JJ, Doros G, Massaro JJ, Garcia-Garcia HM, Bennett J, Roguin A, et al. Ultrathin bioresorbable polymer sirolimus-eluting stents versus thin durable polymer everolimus-eluting stents. J Am Coll Cardiol. 2018;72(25):3287-3297.
doi pubmed - Gupta M, Batra V, Girish MP, Bansal A, Tyagi S. TCT-304 1-year clinical outcomes in patients implanted with biodegradable polymer coated ultrathin strut sirolimus-eluting coronary stent system for the treatment of very long (≥40-mm) lesions. J Am Coll Cardiol. 2019;74:(13_Supplement):B302.
- Rao Prakasa VS, Narayana Rao ASV, Kapardhi PLN, Shah PK, Viswanath R, Mehetre SG, Srivastava AK, et al. Safety and performance of sirolimus-eluting coronary stent system with biodegradable polymer: a retrospective analysis in real-world patient population. World J Cardiov Dis. 2017;7(5):163-173.
- Iniguez A, Chevalier B, Richardt G, Neylon A, Jimenez VA, Kornowski R, Carrie D, et al. Comparison of long-term clinical outcomes in multivessel coronary artery disease patients treated either with bioresoarbable polymer sirolimus-eluting stent or permanent polymer everolimus-eluting stent: 5-year results of the CENTURY II randomized clinical trial. Catheter Cardiovasc Interv. 2020;95(2):175-184.
doi pubmed pmc - Zhang F, Yang J, Qian J, Ge L, Zhou J, Ge J, investigators Fr. Long-term performance of the second-generation cobalt-chromium sirolimus-eluting stents in real-world clinical practice: 3-year clinical outcomes from the prospective multicenter FOCUS registry. J Thorac Dis. 2016;8(7):1601-1610.
doi pubmed pmc - Lemos PA, Chandwani P, Saxena S, Ramachandran PK, Abhyankar A, Campos CM, Marchini JF, et al. Clinical outcomes in 995 unselected real-world patients treated with an ultrathin biodegradable polymer-coated sirolimus-eluting stent: 12-month results from the FLEX Registry. BMJ Open. 2016;6(2):e010028.
doi pubmed pmc - Ajmera P, Pothineni R, Chawla KK, Mantravadi SS, Jariwala P, Vijan V, Vijan V. Twelve months clinical outcomes of ultrathin strut sirolimus-eluting stent in real-world Indian patients with coronary artery disease. Am J Cardiovasc Dis. 2022;12(5):262-271.
pubmed pmc - Pothineni RB, Vijan V, Potdar A, Inamdar MK, Pathak A, Mantravadi SS, Ajmera P. Clinical outcomes of ultrathin biodegradable polymer-coated sirolimus-eluting stents in an all-comer population: One-year results from the T-FLEX registry including high-risk subgroups. Anatol J Cardiol. 2021;25(10):706-715.
doi pubmed pmc - Suwannasom P, Athiksakul S, Thonghong T, Lertsuwunseri V, Chaipromprasit J, Srimahachota S, Udayachalerm W, et al. Clinical outcomes of an ultrathin-strut sirolimus-eluting stent in all-comers population: Thailand Orsiro registry. BMC Cardiovasc Disord. 2021;21(1):501.
doi pubmed pmc - Garg N, Chawla R, Tandon V, Garg D, Parshottam N, Vani P, Neuss M. Real-world five-year outcomes of FlexyRap((R)) cobalt-chromium rapamycin-eluting stents with biodegradable polymer in patients with de-novo coronary artery disease. World J Cardiol. 2023;15(3):84-94.
doi pubmed pmc - Youn YJ, Yoo SY, Lee JW, Ahn SG, Lee SH, Yoon J, Park JH, et al. Safety and efficacy of a new ultrathin sirolimus-eluting stent with abluminal biodegradable polymer in real-world practice. Korean Circ J. 2020;50(4):317-327.
doi pubmed pmc - Kandzari DE, Garcia-Garcia HM, Stoler RC, Wang J, Picone M, Ben-Dor I, Garcia SA. Ultrathin bioresorbable polymer sirolimus-eluting stents in US patients undergoing coronary revascularization: 1-Year outcomes from the BIOFLOW VII trial. Catheter Cardiovasc Interv. 2023;102(3):464-471.
doi pubmed - Maillard L, de Labriolle A, Brasselet C, Faurie B, Durel N, de Poli F, Bosle S, et al. Evaluation of the safety and efficacy of the Cobra PzF NanoCoated coronary stent in routine, consecutive, prospective, and high-risk patients: The e-Cobra study. Catheter Cardiovasc Interv. 2021;98(1):45-54.
doi pubmed - Eberli FR, Stoll HP, Urban P, Morice MC, Brunel P, Maillard L, Lipiecki J, et al. Polymer-free Biolimus-A9 coated thin strut stents for patients at high bleeding risk 1-year results from the LEADERS FREE III study. Catheter Cardiovasc Interv. 2022;99(3):593-600.
doi pubmed pmc - Iglesias JF, Roffi M, Degrauwe S, Secco GG, Aminian A, Windecker S, Pilgrim T. Orsiro cobalt-chromium sirolimus-eluting stent: present and future perspectives. Expert Rev Med Devices. 2017;14(10):773-788.
doi pubmed - Kastrati A, Mehilli J, Dirschinger J, Dotzer F, Schuhlen H, Neumann FJ, Fleckenstein M, et al. Intracoronary stenting and angiographic results strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Vestn Rentgenol Radiol. 2012;(2):52-60.
pubmed - Pache J, Kastrati A, Mehilli J, Schuhlen H, Dotzer F, Hausleiter J, Fleckenstein M, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol. 2003;41(8):1283-1288.
doi pubmed - Lee DH, de la Torre Hernandez JM. The newest generation of drug-eluting stents and beyond. Eur Cardiol. 2018;13(1):54-59.
doi pubmed pmc - Ormiston JA, Webber B, Ubod B, White J, Webster MW. Stent longitudinal strength assessed using point compression: insights from a second-generation, clinically related bench test. Circ Cardiovasc Interv. 2014;7(1):62-69.
doi pubmed - Lee SJ, Choi DW, Suh Y, Hong SJ, Ahn CM, Kim JS, Kim BK, et al. Long-term clinical outcomes between biodegradable and durable polymer drug-eluting stents: a nationwide cohort study. Front Cardiovasc Med. 2022;9:873114.
doi pubmed pmc - El-Hayek G, Bangalore S, Casso Dominguez A, Devireddy C, Jaber W, Kumar G, Mavromatis K, et al. Meta-analysis of randomized clinical trials comparing biodegradable polymer drug-eluting stent to second-generation durable polymer drug-eluting stents. JACC Cardiovasc Interv. 2017;10(5):462-473.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


