| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 14, Number 5, October 2023, pages 396-402
The Value of Left Internal Mammary Artery Flow Velocity in Predicting the Prognosis of Patients After Coronary Artery Bypass Grafting
Feng Wei Guoa, Hong Chenb, Ya Ling Dongb, Jia Nan Shangb, Li Tao Ruanb, Yang Yana, c, Yan Songb, c
aDepartment of Cardiovascular Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi 710061, China
bDepartment of Ultrasound, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi 710061, China
cCorresponding Author: Yan Song, Department of Ultrasound, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi 710061, China; Yang Yan, Department of Cardiovascular Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi 710061, China
Manuscript submitted August 31, 2023, accepted October 10, 2023, published online October 21, 2023
Short title: LIMAV in Predicting Survival After CABG
doi: https://doi.org/10.14740/cr1566
| Abstract | ▴Top |
Background: The purpose of this study was to explore the value of the left internal mammary artery flow velocity (LIMAV) measured by ultrasound before coronary artery bypass grafting (CABG) in predicting the prognosis of patients after left internal mammary artery (LIMA) bypass grafting.
Methods: One hundred and four patients who underwent CABG with LIMA as the bridge vessel in the cardiovascular surgery department of our hospital between May 2018 and June 2019 were selected. All patients underwent transthoracic Doppler ultrasonography to measure LIMAV preoperatively. Intraoperatively, mean graft flow (MGF) and pulsatility index (PI) of the LIMA bridge were measured using transit time flow measurement (TTFM). The primary endpoint event in this study was cardiac death within 18 months after surgery.
Results: The Cox survival analysis showed that the MGF, the LIMAV and left ventricular ejection fraction (LVEF) were risk factors for death after CABG. The cut-offs of MGF, LIMAV and LVEF for the prediction of death after CABG were ≤ 14 mL/min (area under the curve (AUC): 0.830; sensitivity: 100%; specificity: 65.6%), ≤ 60 cm/s (AUC: 0.759; sensitivity: 65.5%; specificity: 85.3%), and ≤ 44% (AUC: 0.724; sensitivity: 50%; specificity: 88.5%), respectively. Compared with the use of MGF, MGF + LIMAV, combination of the MGF + LIMAV + LVEF (AUC: 0.929; sensitivity: 100%; specificity: 81.1%) resulted in a stronger predictive value (MGF vs. MGF + LIMAV + LVEF: P = 0.02).
Conclusion: LIMAV measured by preoperative transthoracic ultrasound combined with intraoperative MGF and LVEF may have a greater value in predicting patients’ risk of cardiac death after CABG.
Keywords: Coronary artery bypass grafts surgery; Artery; Echocardiography; Internal mammary artery
| Introduction | ▴Top |
Coronary artery bypass grafting (CABG) is one of the most effective methods for the treatment of coronary atherosclerotic heart disease. The internal mammary artery (IMA) is the preferred bridge vessel for CABG because of its low variability and high long-term patency rate [1]. Clinical studies have found that the presence of varying degrees of stenosis in the subclavian artery can lead to reduced flow velocity in the IMA. Patients who choose the ipsilateral IMA as the bridge vessel may have low flow velocity in the bridge vessel after surgery, which may again cause angina and other symptoms of myocardial ischemia [2], even though intraoperative transit time flow measurement (TTFM) did not reveal significant indications of poor bridge vessel function.
At this stage in most of our patients, angiography was not performed prior to CABG to assess the presence of lesions and reduced flow velocity in the left internal mammary artery (LIMA). However, during the procedure, we have also faced cases in which the IMA had a lesion that was not used for bypass, which in turn required bypass grafting using the saphenous vein or radial artery, the procedure that may have led to an unnecessary increase in operative time. Therefore, as early as 2009, a review was published suggesting the need for noninvasive assessment of IMA patency prior to coronary artery surgery [3]. Digital subtraction angiography (DSA) is the gold standard for assessing the vascular structure of the IMAs; however, its invasive nature has greatly limited its clinical use [4]. Although multidetector computed tomography (MDCT) can also accurately assess the vascular structure of the IMAs, it is not suitable for routine pre-CABG because of its radioactive nature and high cost [5]. Color Doppler ultrasound is the most commonly used examination method to assess vascular lesions and flow velocities because of its convenience, practicality, non-radiation and repeatable operation. However, studies using ultrasound to evaluate the IMA before CABG are rarely reported. Therefore, the purpose of this study was to explore the value of the left internal mammary artery flow velocity (LIMAV) measured by ultrasound before CABG in predicting the prognosis of patients after LIMA bypass grafting.
| Materials and Methods | ▴Top |
Patients
This study was a single-center retrospective observational study. This study retrospectively analyzed patients who underwent CABG at our hospital from May 2018 to June 2019. Eighty-one patients (77.9%) were male, with a mean age of 61.18 ± 8.86 years. All patients with coronary artery disease were free of comorbid heart valve disease or other organic heart disease. All patients were bypassed using the LIMA with or without saphenous vein bypass. Inclusion criteria were: 1) no significant stenosis or occlusion in preoperative transthoracic bilateral IMA ultrasonography; 2) non-emergency surgery. Exclusion criteria were: LIMA was extensively diseased, had obvious sign of hematoma, or was damaged in any way that could potentially adversely affect flow. We finally included 104 patients. The primary endpoint event in this study was cardiac death within 18 months after surgery. This study was approved by the IRB of the First Affiliated Hospital of Xi’an Jiaotong University. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Ultrasound of IMA
Color Doppler ultrasound diagnostic instrument (Philips CX50, Philips Medical Devices Group, the Netherlands) equipped with a linear array probe at 7 - 10 MHz was used. All patients perfected bilateral IMA ultrasonography before the procedure to clarify the presence of stenosis or plaque formation in the vessel (discarded for those with stenosis or plaque). The diameter and flow velocity parameters of the LIMA were also obtained (Fig. 1a).
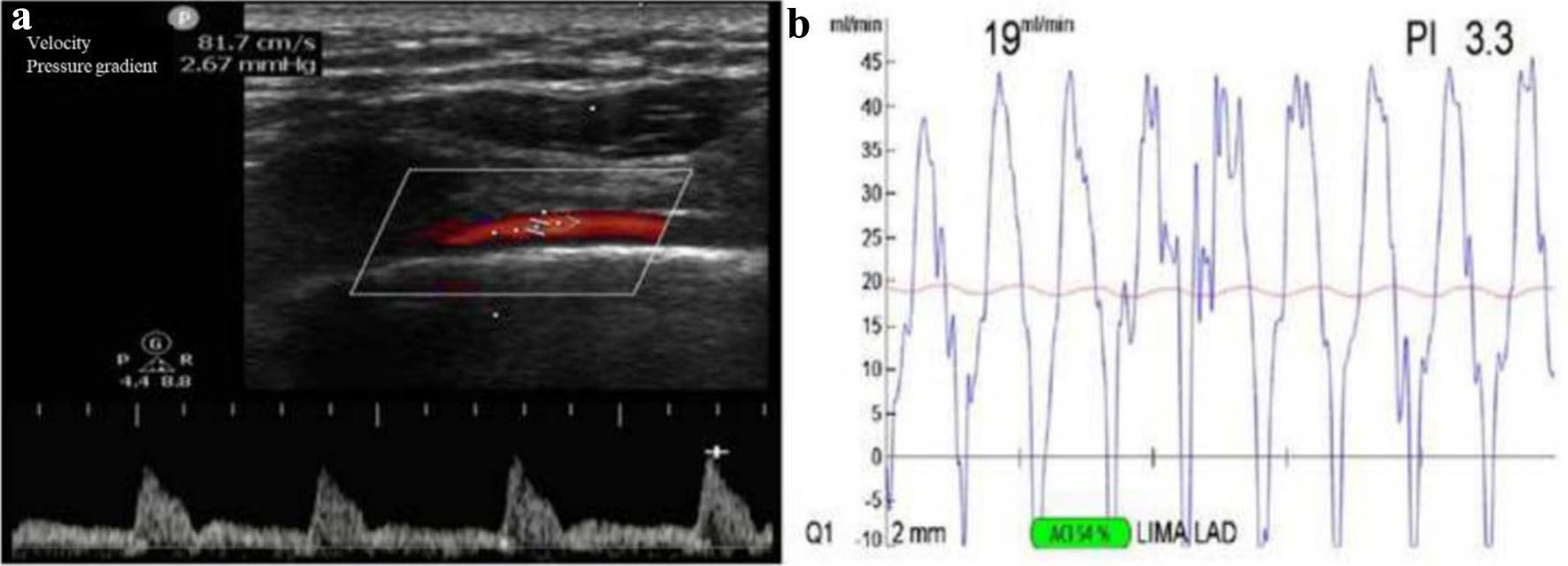 Click for large image | Figure 1. The LIMAV measured by preoperative transthoracic ultrasound and intraoperative MGF. (a) Preoperative LIMAV was 81.7 cm/s. (b) Intraoperative MGF was 19 mL/min. LIMAV: left internal mammary artery flow velocity; MGF: mean graft flow. |
The patient was asked to lie in a supine position with the shoulders elevated and the head slightly tilted back so that the left side of the neck and shoulder could be fully exposed. The probe was placed in the left supraclavicular fossa to make a transverse cut and probe the long axis of the subclavian artery, then the probe was rotated by 90% and slid inward and outward to reveal the beginning of the IMA on the opposite side of the inferior wall of the subclavian artery, that is, the beginning of the vertebral artery. The diameter of the LIMA was measured at the third intercostal space cross-section of the chest wall. Flow velocity was measured in a long-axis view of the IMA in the third intercostal segment (Fig. 1a). All examinations were performed by the same senior ultrasonographer.
Echocardiography
Color Doppler ultrasound diagnostic instrument (Philips CX50, Philips Medical Devices Group, the Netherlands) equipped with a cardiac probe at 1 - 5 MHz was used. We measured overall left ventricular systolic function using the biplane Simpson’s method according to the American Society of Echocardiography (ASE) guidelines [6]. The left ventricular ejection fraction (LVEF) was divided into four grades: male: 52-72% (normal range), 41-51% (mild abnormality), 30-40% (moderate abnormality), < 30% (severe abnormality); female: 54-74% (normal range), 41-53% (mild abnormality), 30-40% (moderate abnormality), < 30% (severe abnormality).
TTFM
Intraoperative blood flow and pulsatility index (PI) in the LIMA bridge vessels were measured using TTFM (Medistim VeriQ, Oslo, Norway) [7] (Fig. 1b). TTFM parameters included: 1) mean flow (Q, mean graft flow (MGF)), which is the mean blood flow in the bypass graft vessel; 2) PI value, which is the ratio of the difference between the maximum and minimum blood flow in the graft vessel to the mean flow (PI = (Qmax - Qmin)/Qm).
After all bridge vessels were anastomosed, they were neutralized with fisetin. We waited for the circulation to stabilize. We then selected the appropriate size ultrasound probe (2 or 3) according to the bridge vessel diameter and placed the bridge vessel into the probe near the anastomosis for direct measurement. For unsatisfactory measurements, the surrounding connective tissue can be removed, skeletonized, and measured again. If necessary, the anastomosis and the graft vessel need to be repeatedly examined or even re-anastomosed and measured again. Criteria for determining whether the blood flow in the bridge vessel is satisfactory included: 1) satisfactory coupling (> 50% or more) when measuring the bridge vessel in the IMA; 2) TTFM shows a stable and reproducible flow waveform pattern; 3) the average flow red line is stable at the plateau period after recording the above data.
CABG
Patients were extubated and CABG was performed under general anesthesia. All patients underwent CABG by median thoracotomy. We used low-frequency electroknife to free the IMAs bilaterally, and after systemic heparinization, we dissected the distal IMAs and protected them with poppyine wet gauze. The corresponding target vessel anastomosis was then completed.
Outcome
To be classified as cardiac death, any of the following had to be fulfilled: death caused by myocardial infarction, heart failure, cardiac tamponade, ventricular fibrillation, pulseless electrical activity, atrial fibrillation, and arrhythmia [8].
Statistical analysis
Data were expressed as either mean ± standard deviation (SD), median and interquartile range (25th and 75th percentiles), or frequency (%). Correlation was measured using Spearman correlation analysis. We performed a Cox proportional-hazards regression modeling to study independent predictors of death. Receiver-operating characteristic (ROC) curve analysis was used to determine the optimal cut-off points for LIMAV, MGF and LVEF to predict death. ROC curves were used to compare the values of MGF, MGF + LIMAV, and MGF + LIMAV + LVEF in prediction. A P value of < 0.05 was considered significant. All calculations were processed using the SPSS software package and Medcal software package for Macintosh (SPSS, Chicago, IL, USA).
| Results | ▴Top |
Basic information
Tables 1 and 2 list the basic information and laboratory tests of 104 patients with coronary atherosclerotic heart disease (CAD) combined or not with heart valve disease who were included in the study. All patients underwent CABG. Of the 104 patients, nine (8.7%) had previous acute myocardial infarction (AMI) and seven (6.7%) had previous percutaneous coronary intervention (PCI). AMI occurred in 18 patients (17.3%) at this time, of which AMI complications were as follows: ventricular wall tumor formation, one case (1%), ventricular septum perforation, one case (1%). LVEF results measured by the biplane Simpson method of echocardiography were as follows: normal, 77 cases (74.1%); mildly abnormal, 17 (16.3%); moderately abnormal, 10 (9.6%).
 Click to view | Table 1. Preoperative Baseline Characteristics of CABG Patients (n = 104) |
 Click to view | Table 2. Preoperative and Intraoperative Characteristics of LIMA (n = 104) |
In all 104 CABG patients, the maximum number of bypasses was 8, the minimum was 1, and the average was 3. The LIMA bypass sites were as follows: LIMA-left anterior descending (LAD), 78 cases (75%); LIMA-diagonal branch of coronary artery (DIAG)-LAD, 18 cases (17.2%); LIMA-DIAG, five cases (4.8%); LIMA-left circumflex coronary artery (LCX), one case (1%); LIMA-right branch of coronary artery (RCA), two case (2%). The total number of bridges was 368. Of all 104 CABG patients, intraoperative extracorporeal circulation assistance was performed in 20 cases (19.2%). In all patients, the mean LIMA diameter was 2.38 ± 0.38 mm and mean LIMAV was 78.69 ± 18.81 cm/s, which were measured by ultrasound before CABG. The mean value of MGF measured after CABG was 22.37 ± 15.07 mL/min and the mean value of PI was 2.61 ± 0.89.
Association between the LIMAV and MGF
Correlation analysis showed a correlation between LIMAV and MGF (r = 0.210, P = 0.033), while there was no correlation with other relevant parameters.
Survival analysis
Among the 104 patients, eight patients died over the follow-up period (at the 5th, 5th, 10th, 11th, 11th, 17th, 17th, 18th month, respectively). All eight patients were cardiac death. Among these eight cases, six patients with LIMA-LAD, two patients with LIMA-DIAG, and all these eight patients were single-vessel bypass graft and did not undergo venous bypass grafting.
The Cox survival analysis showed that the MGF (hazard ratio (HR) = 0.748, 95% confidence interval (CI): 0.600 - 0.932, P = 0.010), the LIMAV (HR = 0.922, 95% CI: 0.857 - 0.992, P = 0.029) and LVEF (HR = 0.910, 95% CI: 0.884 - 0.980, P = 0.013) were risk factors for death after CABG (Table 3). It is suggested that the lower of the MGF, the lower of the LIMAV, the lower of the LVEF, the shorter the survival time of the patients.
 Click to view | Table 3. Univariate and Multivariate Cox Regression Analysis for Association Between Death and Various Parameters |
ROC analysis
The optimal cut-off points for the prediction of death after CABG obtained using ROC curve analysis are shown in Table 4. The cut-offs of MGF, LIMAV and LVEF for the prediction of death after CABG were ≤ 14 mL/min (AUC: 0.830), ≤ 60 cm/s (AUC: 0.759) and ≤ 44% (AUC: 0.724), respectively (Table 4).
 Click to view | Table 4. Predictive Value for Variables When Used to Predict Death |
Compared with the use of MGF, MGF + LIMAV, combination of the MGF + LIMAV + LVEF resulted in a stronger predictive value (MGF vs. MGF + LIMAV + LVEF: P = 0.02) (Fig. 2).
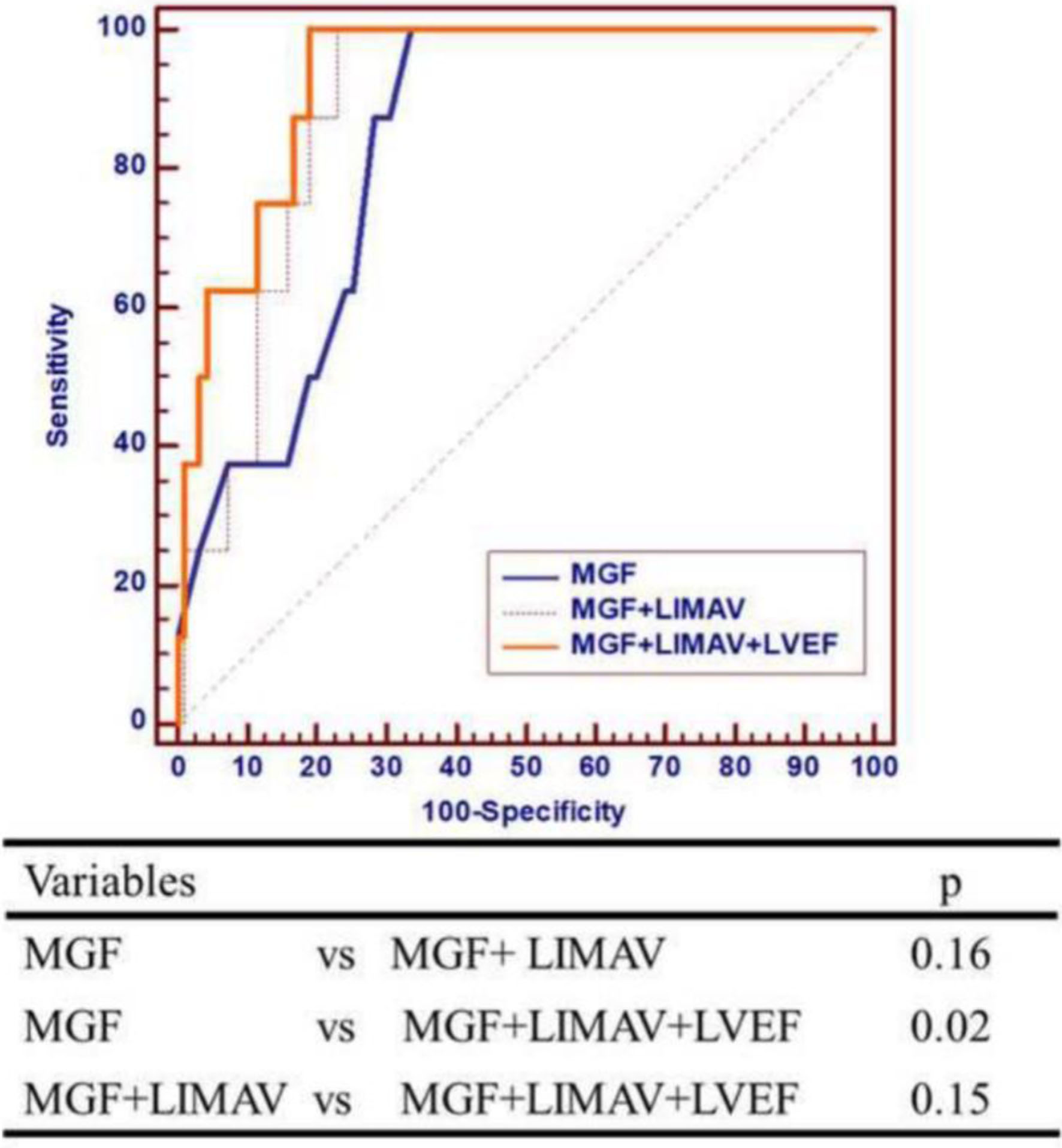 Click for large image | Figure 2. Compared with the use of MGF, MGF + LIMAV, combination of the MGF + LIMAV + LVEF resulted in a stronger predictive value (MGF vs. MGF + LIMAV + LVEF: P = 0.02). LIMAV: left internal mammary artery flow velocity; LVEF: left ventricular ejection fraction; MGF: mean graft flow. |
| Discussion | ▴Top |
In CABG with the IMA as the bridge vessel, if there is stenosis or occlusion of the ipsilateral subclavian artery, the flow velocity of the ipsilateral IMA will be reduced accordingly, which will directly affect the coronary blood supply after CABG, and the symptoms of myocardial ischemia will occur. The possible hemodynamic changes are as follows: stenosis or occlusion at the beginning of the subclavian artery, resulting in reduced flow velocity in the ipsilateral IMA, or low flow velocity due to the direct receipt of blood from the subclavian artery [2]. According to previous reports [9], the lumen of the IMA will gradually become thinner and the intima will gradually thicken under low-flow conditions, thus affecting the blood supply to the bridge vessels. With the prolongation of the disease and the aggravation of subclavian artery stenosis, angina pectoris, myocardial infarction and other symptoms of myocardial ischemia will occur again, which may lead to cardiac death in severe cases.
TTFM has become an important tool for evaluating the blood flow status of bypass graft anastomoses during CABG surgery because of its accuracy, convenience, stability and reproducibility [10, 11], and is now widely used in clinical work in coronary surgery [12]. Factors that may affect TTF include blood viscosity, graft vessel quality and internal diameter size, vascular resistance, own coronary artery internal diameter, resistance, and arterial vasospasm [13].
Tokuda and colleagues [14] evaluated graft patency in 261 grafts and performed a 3-month angiographic follow-up in 123 patients, concluding that TTFM parameters may be a useful predictor of early graft failure. The same group evaluated 104 grafts in 51 patients in a separate study 1 year later with follow-up angiography 1 - 4 years after surgery, and the findings suggested that TTFM provided a good prognostic indicator for intermediate outcomes [13]. Despite the recommendation from the European guidelines for myocardial revascularization [15] to consider MGF below 20 mL/min and PI above 5 as suggestive of an inappropriate graft, those figures remain controversial [11, 16]. Di Giammarco et al concluded that MGF < 15 mL/min is one of the predictors of poor short-term prognosis in patients [17]. Most often, a cut-off value of 15 to 20 mL/min for MGF has been recommended, regardless of the conduit and the anastomosis site [17-19]. In our study, we found that MGF ≤ 14 mL/min was associated with poor prognosis in patients in 18 months, and this cut-off value seems to be lower than those obtained in any previous study.
In our study, we found a positive correlation between preoperative LIMAV and intraoperative MGF. The results of Cox regression analysis showed that MGF, LIMAV and LVEF were also associated with cardiac death in patients after CABG. We also found that MGF combined with LIMAV and LVEF was more effective in predicting all-cause cardiac death after CABG than MGF alone. There was no significant difference in the value of intraoperative MGF combined with LIMAV compared with MGF alone for predicting all-cause mortality. When MGF ≤ 14 mL/min, LIMAV ≤ 60 cm/s and LVEF ≤ 44%, patients are more likely to die of cardiac origin after CABG. In our study, we added the results of LIMAV and LVEF at baseline status to the study. We thought that MGF combined with LIMAV and LVEF may be of greater value in predicting the risk of cardiac death after CABG. To our knowledge, there are fewer studies on the predictive value of MGF combined with LIMAV and LVEF in predicting cardiogenic death after CABG.
Our study has some limitations. Firstly, our study is retrospective and the possibility of bias cannot be excluded. Secondly, all patients who died did not undergo further DSA or computed tomographic angiography although other cardiac diseases were excluded. Thirdly, the number of cases in our study was small and a large number of cases are still needed to confirm our conclusions. Fourthly, only LIMA was studied in this study, but not right internal mammary artery (RIMA). Fifthly, patients with both angina pectoris and obsolete myocardial infarction were included. Obsolete myocardial infarction may affect the vascular bed of the myocardium and thus the graft blood flow.
Conclusion
LIMAV measured by preoperative transthoracic ultrasound combined with intraoperative MGF and LVEF may have a greater value in predicting patients’ risk of cardiac death after CABG.
Acknowledgments
The authors thank Professor Jie Zheng (The clinical research center (CRC), The First Affiliated Hospital of Xi’an Jiaotong University) for statistical guidance. A preprint has previously been published [20].
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Author Contributions
Yan Song, Yang Yan, and Li Tao Ruan conceived and designed the project. Feng Wei Guo and Hong Chen interpreted the results and wrote the manuscript. Ya Ling Dong and Jia Nan Shang collected data, collated the echocardiographic data. All authors reviewed the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Rankin JS, Tuttle RH, Wechsler AS, Teichmann TL, Glower DD, Califf RM. Techniques and benefits of multiple internal mammary artery bypass at 20 years of follow-up. Ann Thorac Surg. 2007;83(3):1008-1014; discussion 1014-1005.
doi pubmed - Sintek M, Coverstone E, Singh J. Coronary subclavian steal syndrome. Curr Opin Cardiol. 2014;29(6):506-513.
doi pubmed - Barbetakis N, Lafaras C, Efstathiou A, Fessatidis I. eComment: Routine preoperative evaluation of the internal mammary artery as conduit for coronary patients. Is it worth? Interact Cardiovasc Thorac Surg. 2009;9(4):727.
doi pubmed - Turkvatan A, Biyikoglu SF, Buyukbayraktar FG, Cumhur T, Duru E, Olcer T, Ulas MM. Noninvasive evaluation of coronary artery bypass grafts and native coronary arteries: is 16-slice multidetector CT useful? Diagn Interv Radiol. 2009;15(1):43-50.
pubmed - Karaman B, Battal B, Bozkurt Y, Bozlar U, Demirkol S, Sahin MA, Tasar M. The anatomic evaluation of the internal mammary artery using multidetector CT angiography. Diagn Interv Radiol. 2012;18(2):215-220.
doi pubmed - Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14.
doi pubmed - Balacumaraswami L, Taggart DP. Intraoperative imaging techniques to assess coronary artery bypass graft patency. Ann Thorac Surg. 2007;83(6):2251-2257.
doi pubmed - Herlitz J, Brandrup-Wognsen G, Caidahl K, Haglid-Evander M, Hartford M, Karlson B, Karlsson T, et al. Cause of death during 13 years after coronary artery bypass grafting with emphasis on cardiac death. Scand Cardiovasc J. 2004;38(5):283-286.
doi pubmed - Barner HB. Remodeling of arterial conduits in coronary grafting. Ann Thorac Surg. 2002;73(4):1341-1345.
doi pubmed - D'Ancona G, Karamanoukian HL, Ricci M, Bergsland J, Salerno TA. Graft patency verification in coronary artery bypass grafting: principles and clinical applications of transit time flow measurement. Angiology. 2000;51(9):725-731.
doi pubmed - Di Giammarco G, Rabozzi R. Can transit-time flow measurement improve graft patency and clinical outcome in patients undergoing coronary artery bypass grafting? Interact Cardiovasc Thorac Surg. 2010;11(5):635-640.
doi pubmed - Niclauss L. Techniques and standards in intraoperative graft verification by transit time flow measurement after coronary artery bypass graft surgery: a critical review. Eur J Cardiothorac Surg. 2017;51(1):26-33.
doi pubmed - Tokuda Y, Song MH, Oshima H, Usui A, Ueda Y. Predicting midterm coronary artery bypass graft failure by intraoperative transit time flow measurement. Ann Thorac Surg. 2008;86(2):532-536.
doi pubmed - Tokuda Y, Song MH, Ueda Y, Usui A, Akita T. Predicting early coronary artery bypass graft failure by intraoperative transit time flow measurement. Ann Thorac Surg. 2007;84(6):1928-1933.
doi pubmed - Kolh P, Windecker S, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2014;46(4):517-592.
doi pubmed - Kieser TM, Rose S, Kowalewski R, Belenkie I. Transit-time flow predicts outcomes in coronary artery bypass graft patients: a series of 1000 consecutive arterial grafts. Eur J Cardiothorac Surg. 2010;38(2):155-162.
doi pubmed - Di Giammarco G, Pano M, Cirmeni S, Pelini P, Vitolla G, Di Mauro M. Predictive value of intraoperative transit-time flow measurement for short-term graft patency in coronary surgery. J Thorac Cardiovasc Surg. 2006;132(3):468-474.
doi pubmed - Quin J, Lucke J, Hattler B, Gupta S, Baltz J, Bishawi M, Almassi GH, et al. Surgeon judgment and utility of transit time flow probes in coronary artery bypass grafting surgery. JAMA Surg. 2014;149(11):1182-1187.
doi pubmed - Di Giammarco G, Canosa C, Foschi M, Rabozzi R, Marinelli D, Masuyama S, Ibrahim BM, et al. Intraoperative graft verification in coronary surgery: increased diagnostic accuracy adding high-resolution epicardial ultrasonography to transit-time flow measurement. Eur J Cardiothorac Surg. 2014;45(3):e41-45.
doi pubmed - Feng-Wei Guo, Hong Chen, Ya-Ling Dong, et al. The value of left internal mammary artery velocity in predicting the prognosis of patients after CABG. Preprint. 2021.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


