| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Case Report
Volume 14, Number 6, December 2023, pages 464-467
Left Anterior Descending Artery Dissection in a Female Patient With History of Chest Radiation Treatment and Separate Ostia of the Left Coronary Arteries
Christos Papageorgioua, b , Vaios Tzifosa
aDepartment of Interventional Cardiology, Henry Dunant Hospital Center, Athens 11526, Greece
bCorresponding Author: Christos Papageorgiou, Department of Interventional Cardiology, Henry Dunant Hospital Center, Athens 11526, Greece
Manuscript submitted December 10, 2023, accepted December 18, 2023, published online December 29, 2023
Short title: A Case of LAD Artery Dissection
doi: https://doi.org/10.14740/cr1603
| Abstract | ▴Top |
Obstructive and flow limiting coronary artery dissections can be a catastrophic clinical scenario, requiring urgent treatment and tailored approach for each case. A 55-year-old female patient, with a history of breast cancer, chest radiation treatments and hypertension presented with episodes of angina and significant area of reversible ischemia on single-photon emission computerized tomography (SPECT). Coronary angiogram revealed separate ostia of the left coronary arteries and three-vessel disease (SYNTAX (Synergy between percutaneous coronary intervention (PCI) with Taxus and Cardiac Surgery) = 15); subsequent full revascularization was achieved successfully with two drug-eluting stents (DES) (mid left anterior descending artery (LAD), left circumflex coronary artery (LCx)) and one drug-coated balloon (posterior descending artery (PDA)). However, after a few hours the patient underwent an urgent second angiography due to ongoing chest pain and electrocardiogram (ECG) changes. Proximal complete occlusion of the anomalous LAD was displayed and a long dissection attributable to an intimal tear following first stent implantation was recorded (well expanded and apposed stent (proximal stent edges were implanted in an unhealthy vessel area infiltrated with fibrotic and calcified plaque) not detectable by conventional angiography). A second 3.5 × 38 mm DES was implanted optimally in the proximal LAD segment and overlapped with the first one, with immediate restoration of the flow and relief of the patient’s symptoms. The patient was discharged symptom free and with recommendation for optimal medical treatment for secondary coronary artery disease (CAD) prevention. Conventional coronary angiography in patients with history of chest radiation treatment might not detect accurately the extent and characteristics of the underlying CAD. Appropriate use of intravascular imaging in these cases secures a safe approach for ambiguous lesions and facilitates treatment of iatrogenic coronary dissections following PCI.
Keywords: Coronary dissection; Radiation-induced CAD; Congenital coronary anomalies; Intravascular imaging; Complications management
| Introduction | ▴Top |
Coronary artery anomalies approximately account for 1-5.64% of the general population [1]. The commonest identified anomaly in patients undergoing coronary angiography is separate left anterior descending artery (LAD) and left circumflex coronary artery (LCx) ostia (1-2%) from the left sinus of Valsalva [2]. Catheter manipulations and cannulation during angiography/angioplasty may carry substantial risks with regards to the anomalous ostia injury and subsequent severe coronary flow limiting dissections [3]. Moreover, predisposing factors such as history of chest radiation treatments might significantly alter the normal architecture of the involved arteries segments, triggering accelerated atherosclerosis and promoting vessel stenosis and extended intimal fibrosis [4, 5].
| Case Report | ▴Top |
A 55-year-old lady presented with episodes of stable angina (Canadian Cardiovascular Society II (CCS II)) for the past 2 months and reversible ischemia (single-photon emission computerized tomography (SPECT) demonstrated) of the anterior, basal-lateral wall and the apex regions (> 10%). From her past medical history, she had arterial hypertension and a history of left breast cancer for which she received 15 sessions of radiation treatments 20 years ago. Coronary angiogram revealed separate ostia of the left coronary artery and three-vessel disease with a SYNTAX (Synergy between percutaneous coronary intervention (PCI) with Taxus and Cardiac Surgery) score of 15. The patient decided to proceed with PCI after being informed of the indicated treatment options.
Management/treatment
Loading with dual antiplatelet treatment (325 mg of aspirin, 600 mg of clopidogrel) and administration of unfractionated heparin (7,000 IU) were initiated. Following the successfully treatment of lesions in the posterior descending artery (PDA) and the LCx with a drug-coated balloon (DCB) and one drug-eluting stents (DES) respectively, we decided to treat an 80% mid LAD angiographically type B1 lesion (Fig. 1). A 6 French extra backup 3.5 guide catheter was used to cannulate the LAD ostium. A 2.5 × 15 mm non-compliant balloon at 12 atm was used for predilation, followed by successful implantation of a 3 × 20 mm DES at 14 atm with excellent angiographic result (Supplementary Material 1, www.cardiologyres.org). However, after a few hours the patient complained of ongoing chest pain and developed new electrocardiogram (ECG) changes suggestive of ischemia in the region of the LAD. A repeat urgent angiography demonstrated severe occlusion of the proximal LAD with thrombolysis in myocardial infarction (TIMI) 1 flow (Fig. 2a). Following careful engagement of the LAD separate ostium with a hydrophilic wire, intravascular ultrasound (IVUS) imaging was used to interrogate the vessel and confirmed the diagnosis of proximal-mid dissection of the vessel with identification of the true and false lumens (Fig. 2b, c; Supplementary Material 2, www.cardiologyres.org). The previously implanted stent was well expanded and apposed. Direct stenting of the proximal segment with one DES of 3.5 × 38 mm in the true lumen of the vessel overlapped with the first stent was performed subsequently with immediate restoration of the flow and relief of patient’s symptoms (Fig. 3). A repeat IVUS run confirmed the good apposition and expansion of the second stent (Supplementary Material 3, www.cardiologyres.org). The patient had an uneventful rest of stay and was discharged symptom free, with an indication for a follow-up nuclear imaging study at 1 year or a coronary computer tomography angiography (in order to assess the long-term result following our intervention with a dedicated coronary imaging modality) in case of recurrent symptoms, and recommendation for optimal medical treatment for secondary prevention (antiplatelet treatment, adequate blood pressure management and drastic low-density lipoprotein cholesterol (LDL-C) reduction).
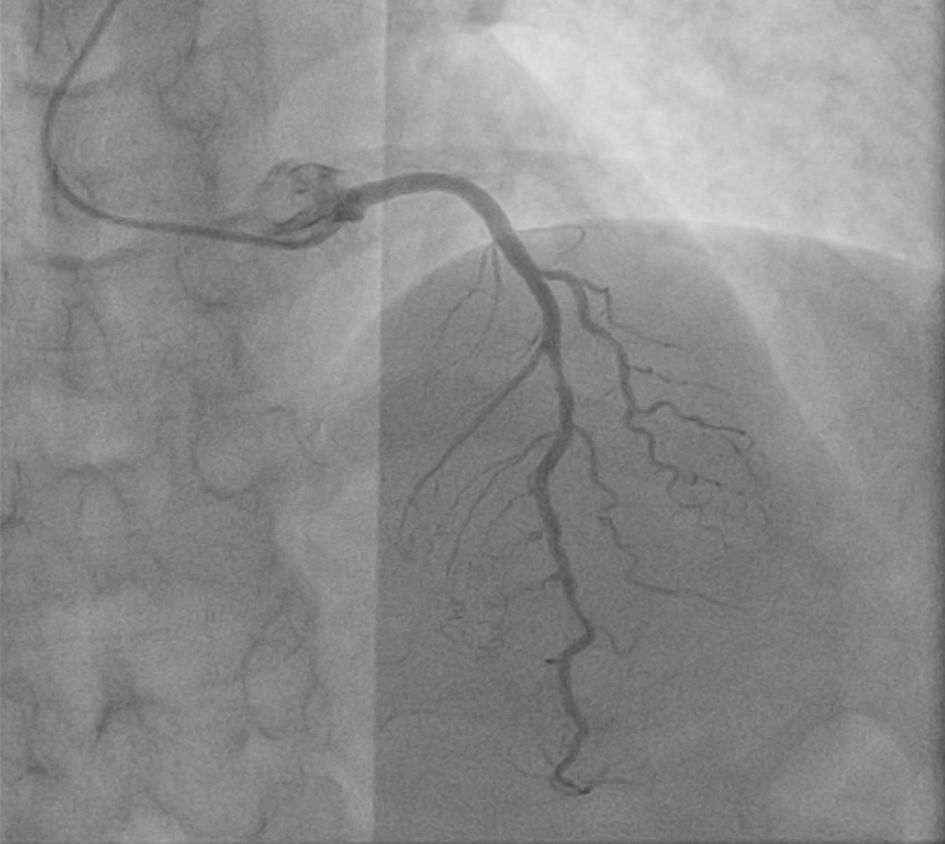 Click for large image | Figure 1. LAD of anomalous origin (separate ostium) with a mid-80% type B1 angiographic lesion. LAD: left anterior descending artery. |
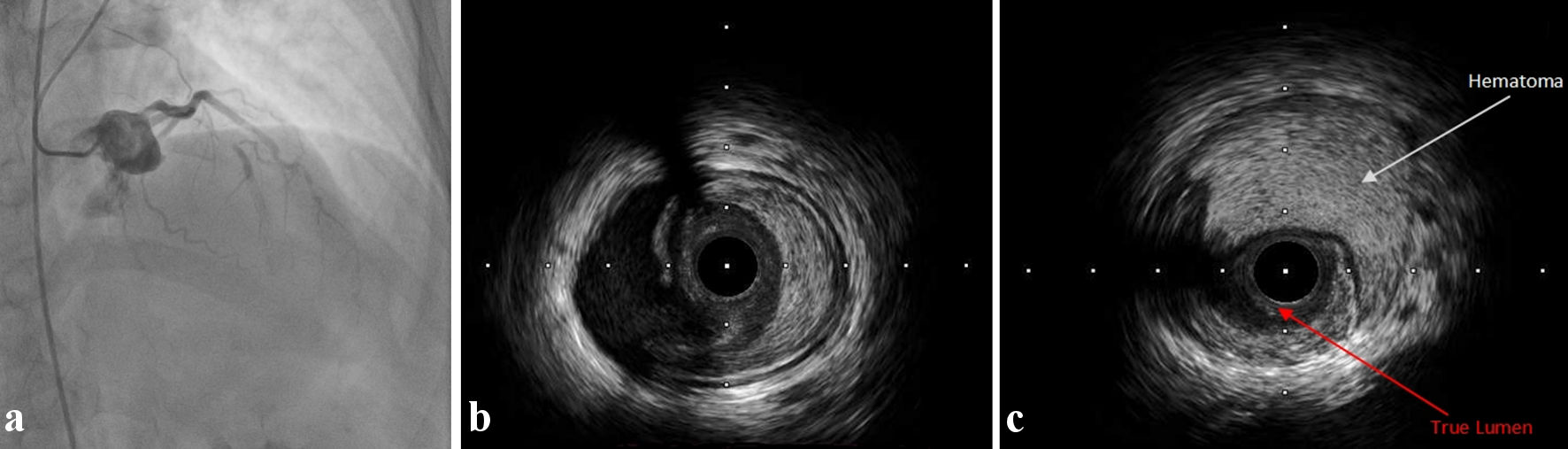 Click for large image | Figure 2. (a) Proximal occlusion of the previously stented LAD. (b) Intimal tear in mid LAD involving the media and fibrotic plaque. (c) Large area of hematoma and identification of the compressed true vessel lumen. LAD: left anterior descending artery. |
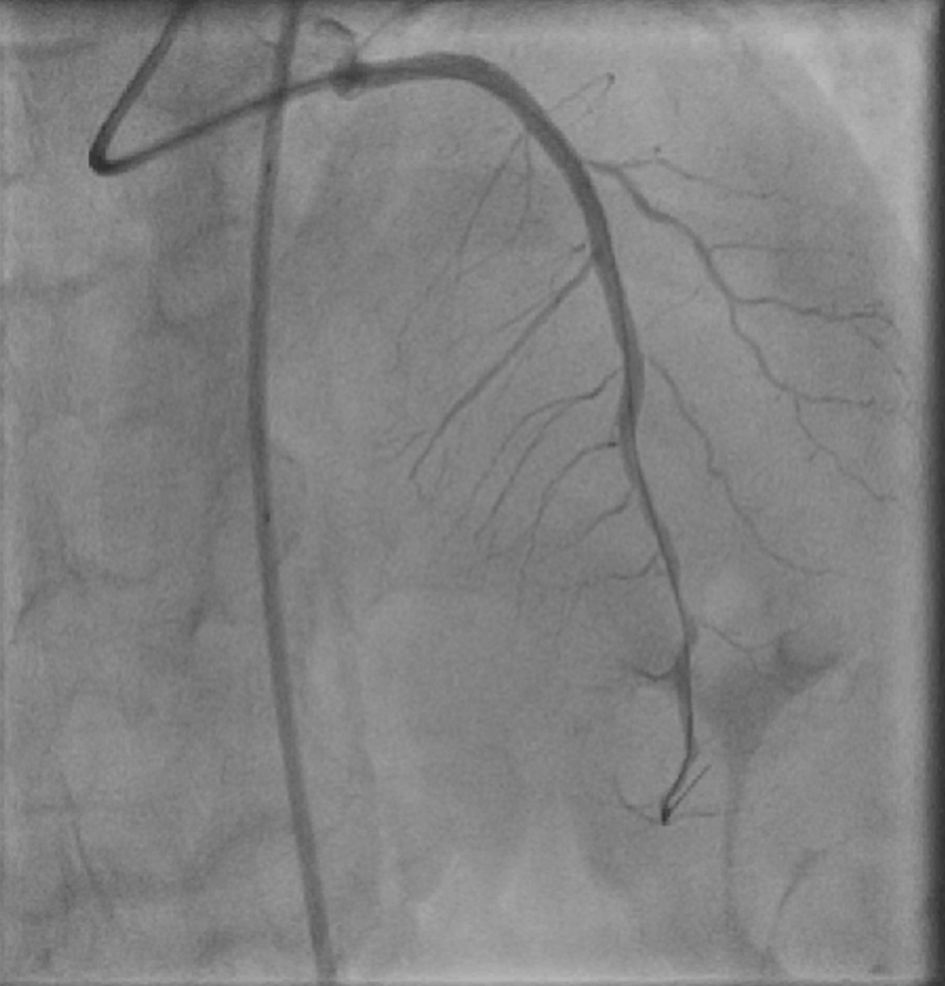 Click for large image | Figure 3. Final angiographic result after the implantation of one DES 3.5 × 38 mm at the ostial part of the vessel, overlapped with the previously implanted stent. DES: drug-eluting stents. |
The present work is compliant with the ethical requirements of the Declaration of Helsinki, regarding research involving human subjects.
| Discussion | ▴Top |
History of chest radiation treatment is an important predisposing factor for coronary artery disease (CAD) in cancer patients with a reported prevalence of 85% [6]. Additional risk factors for CAD in this group of patients include young age, history of cardiovascular disease, the use of cardiotoxic agents for chemotherapy (e.g., anthracyclines), hypertension, dyslipidemia, diabetes, smoking, and family history of CAD. Technical aspects of radiation treatment sessions have also been implicated to CAD progression (accumulative dose reception, tissue/fraction radiation dosage, amount of heart muscle affected and the exposure of the coronary arteries to the radiation beam) [7-10].
Left-sided breast radiation has been correlated mostly with LAD involvement. Radiation beams induct endothelial injury in the coronaries leading to the production of free radicals and a subsequent activation of the inflammatory cascade. The activated inflammatory cytokines subsequently promote platelet activation, vessel wall erosion/rupture and vessel thrombosis [7]. Cardinal feature of the radiation-induced CAD is the infiltration of the injured intima area with fibroblasts [7, 9]. Lesions seen with radiation are located often in the proximal segments of the vessel and frequently involve the coronary ostia, while symptom manifestation of CAD can occur after years following treatment [4, 5]. Cannulation maneuvers during angiography/angioplasty of congenital anomalous coronaries compromised by radiation and the negotiation of proximal lesions in this context can significantly increase the risk of vessel injury and subsequent ostial dissections [3, 11].
In our case and after the patient manifested symptoms and ECG changes related to the LAD region (following the first intervention), we considered initially stent thrombosis vs. missed iatrogenic catheter-induced LAD dissection (considering the congenital anomalous anatomy of the patient). The clinical scenario of the present case however was more suggestive of an iatrogenic coronary dissection after the first stent implantation in the mid part of the LAD. IVUS interrogation of the ostial LAD segment before the second DES implantation did not reveal dissected areas indicative of an iatrogenic catheter-induced dissection, which would have happened during previous ostium cannulation (Supplementary Material 3, www.cardiologyres.org).
However, stenting an extended fibrotic lesion (in a vessel prone to injury and with damaged architecture due to radiation) with the proximal stent edges implanted in areas with significant plaque burden, resulted to an intimal tear (in the mid part of the vessel) and subsequent dissection with media involvement, not detectable angiographically immediately after PCI, which propagated gradually.
The role of intravascular imaging in cases of coronary dissections has been thoroughly documented in literature [12-14]. In this case, its use was critical as it facilitated the clarification of the correct diagnosis (dissection vs. stent thrombosis) and guided the safe negotiation of the vessel’s true lumen for a successful second stent implantation.
The importance of intravascular imaging-guided PCI with respect to the reduced incidence of target vessel failure, stent thrombosis, cardiac death, and target vessel-related myocardial infarction in patients undergoing DES implantation has also been well described in numerous randomized trials [15, 16]. Correct initial identification of target lesion characteristics, detailed assessment of vessel lumen areas and lengths of the diseased segments facilitate adequate lesion preparation and optimal implantation of DES (when indicated) leading to superior clinical and angiographical results.
It is our notion that interrogation of the initial mid LAD lesion with the use of intravascular imaging and before proceeding with angioplasty could probably have prevented this type of complication.
Conclusions
Patients with history of chest radiation treatment presenting with angina might carry significant intracoronary atheromatic burden that is not detectable with conventional angiography alone. The prompt use of intravascular imaging guidance remains crucial in this context, with respect to the interrogation and treatment of angiographically ambiguous lesions, discerning underlying coronary pathologies and leading to more favorable clinical outcomes.
Learning points
Radiation treatments of the mediastinum or the left breast side can substantially accelerate atherosclerosis of the coronary arteries involved with extended disease even after years following treatment.
In this regard intravascular negotiation of the affected vessel’s lesions requires proper tissue characterization and vessel areas dimensions for the selection of the most appropriate treatment strategy.
PCI in patients with CAD and separate ostia of the left coronary arteries requires vigilance with respect to catheter manipulation and avoidance of iatrogenic dissections of the ostia during catheter engagement.
Complications following coronary interventions, such as obstructive and flow limiting dissections (amenable to PCI), need immediate treatment with the aid of intravascular imaging modalities for the correct true lumen vessel identification and optimal DES implantation.
| Supplementary Material | ▴Top |
Suppl 1. Immediate angiogram post initial PCI.
Suppl 2. Initial intravascular imaging assessment of the LAD.
Suppl 3. Intravascular imaging assessment of the LAD following the second PCI.
Financial Disclosure
No funding was received from any source for the presented work.
Conflict of Interest
The authors would like to declare no conflict of interest.
Informed Consent
Written consent was obtained from the patient.
Author Contributions
CP, VT designed, interpreted, conceptualized, and wrote the present work. Both authors contributed to the final review of this case report.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Angelini P. Coronary artery anomalies: an entity in search of an identity. Circulation. 2007;115(10):1296-1305.
doi pubmed - Ben-Dor I, Weissman G, Rogers T, Slack M, Pichard A, Ben-Dor N, Hashim H, et al. Catheter selection and angiographic views for anomalous coronary arteries: a practical guide. JACC Cardiovasc Interv. 2021;14(9):995-1008.
doi pubmed - Kimura T, Nishibori Y, Miki K, Nishian K, Fujita K, Takada M, Maruyama T. Catheter-induced aortocoronary dissection at the ostium of anomalous left coronary artery during percutaneous coronary intervention for acute inferior myocardial infarction. J Cardiol Cases. 2018;17(3):73-76.
doi pubmed pmc - van Rosendael AR, Daniels LA, Dimitriu-Leen AC, Smit JM, van Rosendael PJ, Schalij MJ, Bax JJ, et al. Different manifestation of irradiation induced coronary artery disease detected with coronary computed tomography compared with matched non-irradiated controls. Radiother Oncol. 2017;125(1):55-61.
doi pubmed - Cuomo JR, Javaheri SP, Sharma GK, Kapoor D, Berman AE, Weintraub NL. How to prevent and manage radiation-induced coronary artery disease. Heart. 2018;104(20):1647-1653.
doi pubmed pmc - Chang HM, Okwuosa TM, Scarabelli T, Moudgil R, Yeh ETH. Cardiovascular Complications of Cancer Therapy: Best Practices in Diagnosis, Prevention, and Management: Part 2. J Am Coll Cardiol. 2017;70(20):2552-2565.
doi pubmed pmc - Belzile-Dugas E, Eisenberg MJ. Radiation-induced cardiovascular disease: review of an underrecognized pathology. J Am Heart Assoc. 2021;10(18):e021686.
doi pubmed pmc - Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, Correa C, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987-998.
doi pubmed - van Nimwegen FA, Schaapveld M, Cutter DJ, Janus CP, Krol AD, Hauptmann M, Kooijman K, et al. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. 2016;34(3):235-243.
doi pubmed - Papageorgiou C, Andrikopoulou A, Dimopoulos MA, Zagouri F. Cardiovascular toxicity of breast cancer treatment: an update. Cancer Chemother Pharmacol. 2021;88(1):15-24.
doi pubmed - Cohen MG, Tolleson TR, Peter RH, Harrison JK, Sketch MH, Jr. Successful percutaneous coronary intervention with stent implantation in anomalous right coronary arteries arising from the left sinus of valsalva: a report of two cases. Catheter Cardiovasc Interv. 2002;55(1):105-108.
doi pubmed - Janssen E, de Leeuw PW, Maas A. Spontaneous coronary artery dissections and associated predisposing factors: a narrative review. Neth Heart J. 2019;27(5):246-251.
doi pubmed pmc - Macaya F, Salazar CH, Perez-Vizcayno MJ, Salinas P, Jimenez-Quevedo P, Nombela-Franco L, Del Trigo M, et al. Feasibility and safety of intracoronary imaging for diagnosing spontaneous coronary artery dissection. JACC Cardiovasc Imaging. 2019;12(4):763-764.
doi pubmed - Macaya F, Salinas P, Gonzalo N, Fernandez-Ortiz A, Macaya C, Escaned J. Spontaneous coronary artery dissection: contemporary aspects of diagnosis and patient management. Open Heart. 2018;5(2):e000884.
doi pubmed pmc - Gao XF, Ge Z, Kong XQ, Kan J, Han L, Lu S, Tian NL, et al. 3-Year Outcomes of the ULTIMATE trial comparing intravascular ultrasound versus angiography-guided drug-eluting stent implantation. JACC Cardiovasc Interv. 2021;14(3):247-257.
doi pubmed - Lee JM, Choi KH, Song YB, Lee JY, Lee SJ, Lee SY, Kim SM, et al. Intravascular imaging-guided or angiography-guided complex PCI. N Engl J Med. 2023;388(18):1668-1679.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


