| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 15, Number 3, June 2024, pages 169-178
Long-Term Safety and Performance of BioMime™ Morph Sirolimus-Eluting Coronary Stent System for Very Long Coronary Lesions
Department of Cardiology, KLES Dr. Prabhakar Kore Hospital & Medical Research Centre, Nehru Nagar, Belagavi 590010, Karnataka, India
Manuscript submitted February 21, 2024, accepted April 8, 2024, published online June 25, 2024
Short title: BioMime™ for Very Long Coronary Lesions
doi: https://doi.org/10.14740/cr1626
| Abstract | ▴Top |
Background: The use of multiple overlapping stents for long lesions in tapered coronary arteries has been associated with poor outcomes. This study was conducted to evaluate the 3-year safety and performance of the BioMime™ Morph sirolimus-eluting stent (SES) in very long (length 30 to ≤ 56 mm) coronary lesions in native coronary arteries with a reference vessel diameter of 2.25 to 3.50 mm.
Methods: This was a prospective, single-center, observational, real-world, post-marketing surveillance study. Eligible patients were implanted with BioMime™ Morph SES. Patients were followed up at 6, 12, 24, and 36 months.
Results: A total of 88 patients were enrolled in the study. The mean age was 58.72 ± 10.10 years and 82.95% were male. Most patients had angina (81.82%) and ischemic heart disease (78.41%), and there was a high prevalence of comorbidities like diabetes mellitus (59.09%), and hypertension (54.55%). A total of 92 long coronary de novo lesions were treated with BioMime™ Morph SES with an average stent length of 45.54 ± 10.20 mm. Device and procedural success rates were 100%. One patient died at 30 days and one case of myocardial infarction was recorded. The cumulative rates of major adverse cardiovascular events (MACEs) at 6, 12, 24, and 36 months were 3.41%, 6.82%, 7.95%, and 7.95%, respectively. There were no cases of stent thrombosis (ST), ischemia-driven target vessel revascularization, or ischemia-driven target lesion revascularization until 36 months of follow-up.
Conclusion: BioMime™ Morph SES showed favorable outcomes up to 3 years in treating very long coronary lesions in native coronary arteries, as demonstrated by an acceptable rate of MACEs and absence of ST, based on clinical outcomes up to 3 years.
Keywords: BioMime Morph; De novo lesions; Drug-eluting stent; Stent thrombosis; Tapered stent; Target vessel failure; Long coronary lesions; Tapering coronary vessels
| Introduction | ▴Top |
Long coronary vessels are prone to natural tapering. Stenosis or occlusions in major parts of a long coronary vessel can pose a challenge in selecting the optimal stent size during percutaneous coronary intervention (PCI) [1]. Tapering is defined as the ratio of the area change to the vessel length [2]. Studies have shown that tapering of coronary arteries is common. Banka et al assessed the degree of taper between 1 cm proximal and distal to the stenosis. They found that 23% of arteries showed > 1 mm taper, 19% of arteries showed 0.5 - 0.99 mm taper, and reverse tapering was seen in 8% of arteries [3]. Similarly, Zhang et al showed that in Asians, the left anterior descending (LAD) artery had an average diameter of 3.92 mm at the origin and 2.10 mm at the distal end, with a 7.7% decrease in the ratio. The average diameter of the left circumflex artery (LCx) was 3.57 mm at the origin and 2.10 mm at the distal end, with a 9.7% decrease in the ratio, and the average diameter of the right coronary artery (RCA) was 3.97 mm at the origin and 2.15 mm at the distal end, with a 5.1% decrease in the ratio [4]. Angiographic data have shown that LAD and RCA taper approximately 14% and 9%, respectively, along their lengths [5].
Contemporary drug-eluting stents (DESs) are commonly used for PCI in long-tapered segments. However, in such cases, there are often considerable discrepancies between the proximal and distal parts of the targeted lesion. Hence, the optimal stent size must be selected according to the distal diameter of the treated segment. However, this leads to a mismatch between the stent size and the proximal diameter of the vessel, which requires post-dilatation using larger balloons to ensure optimal strut apposition [6]. Different stent model designs can have a critical impact on overexpansion results [7]. Incomplete stent apposition is known to increase the risk of in-stent restenosis and stent thrombosis (ST) [8].
Malapposition of stents due to incomplete stent expansion is a predictor of adverse outcomes. On the contrary, extensive overexpansion approaching the physical limits of the stent might affect the mechanical stiffness of the stent and the drug delivery process, limiting the performance of the device [9-11]. In the overexpanded regions, the increased cell diameter can cause a reduction in drug elution per mm, which can lead to a higher risk of excessive neointimal proliferation [6]. Further, there is an increased risk of damage to the drug-coating or the detachment of debris leading to higher rates of thrombosis and inflammation, with neointimal reactions, after overexpansion of the stent [12]. Hence, some clinicians deploy multiple overlapping stents instead of a single long stent. However, studies have shown that stent overlapping is associated with delayed healing and increased inflammation at the site of deployment, which ultimately results in impaired angiographic and long-term clinical outcomes, including death or myocardial infarction (MI) [13].
A dedicated long-tapered DES could overcome the challenges of stenting tapered coronary arteries. It has been reported that a single long-tapered BioMime™ Morph SES system is often adequate for treating a long, diffuse lesion in tapered arteries thereby avoiding the risks associated with multiple stenting and stent overlapping [14]. This stent system is designed to be deployed across single long lesions in a tapering coronary artery. It can be used for de novo lesions with lengths 30 to ≤ 56 mm in coronary arteries like the LAD and RCA. Several previous studies have reported the procedural success rates and 1-year safety and efficacy outcomes of this stent system for long coronary lesions [14-22].
This study was conducted to evaluate the 3-year safety and performance of the aforementioned BioMime™ Morph SES in very long (length 30 to ≤ 56 mm) coronary lesions in native coronary arteries with reference vessel diameters of 2.25 to 3.50 mm.
| Materials and Methods | ▴Top |
Study design and patient population
This was a prospective, single-arm, single-center, observational, post-marketing surveillance study to evaluate the safety and performance of the BioMime™ Morph SES in very long (length 30 to ≤ 56 mm) coronary lesions in native coronary arteries with reference vessel diameter of 2.25 to 3.50 mm in real-world settings.
Eligibility criteria
The inclusion criteria were age ≥ 18 years, significant native coronary artery stenosis (> 50% by visual estimate) with lesion length of 30 to ≤ 56 mm. Patients with contraindication to any of the medications including aspirin, heparin, clopidogrel, cobalt chromium, contrast agents, or sirolimus, those participating in any other drug or device investigational study, or females with ongoing pregnancy or lactation were excluded from the study.
Ethics statement
This study was conducted according to the ICH standards for clinical research including ICH-E6 (Good Clinical Practice) and ICH E3 (Study Reporting); ISO 14155 standards for the conduct of the study. The Institutional Review Board and Independent Ethics Committee provided approval for the study (Ref: KLEU/EC/2016-17/D-3896, dated: January 10, 2017). The study was registered on the National Institute of Medical Statistics portal of the Indian Council of Medical Research, Clinical Trials Registry - India (CTRI) (CTRI number: CTRI/2017/03/008167).
Study device
BioMime™ Morph SES is a novel, ultra-thin (65 µm) balloon expandable sirolimus-eluting long-tapered stent for the treatment of long coronary lesions. It consists of an L605 cobalt-chromium alloy platform coated with biodegradable polymers poly (L-lactide) (PLLA), 50/50 poly (D,L-lactide-co-glycoside) (PLGA). This L605 cobalt-chromium long stent mounted on a long and tapered rapid-exchange percutaneous transluminal coronary angioplasty (PTCA) balloon catheter (Xpedient™/Mozec™) between two platinum-iridium radiopaque marker bands placed on the inner lumen within the balloon segment. Long and tapered sizes of the PTCA balloon catheters are specially designed to suit the anatomically tapering arteries. The balloon tapers from the proximal to the distal end with approximately 0.5 mm taper and the stent remains expanded after deflation of the balloon. BioMime Morph device is depicted in Figure 1.
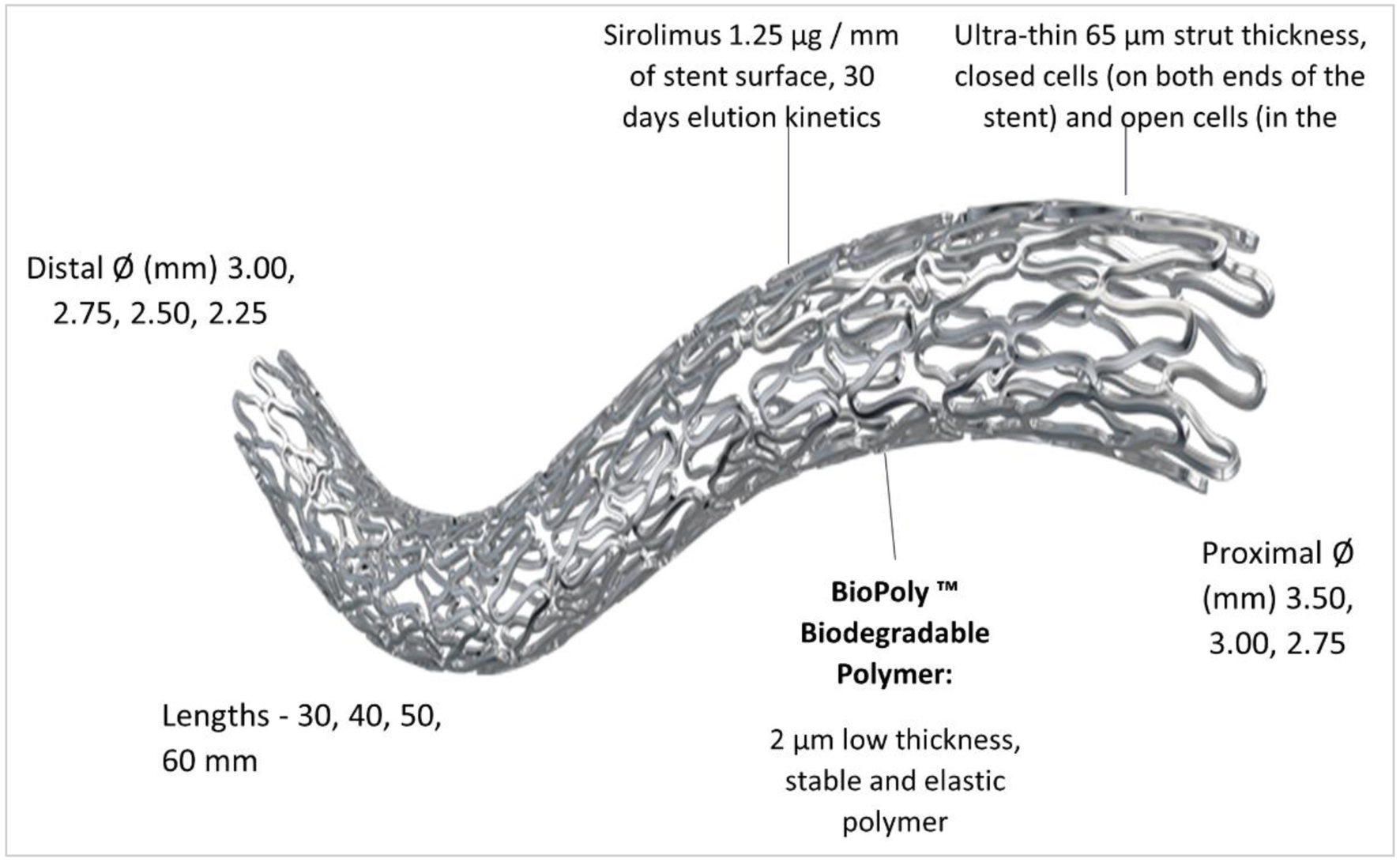 Click for large image | Figure 1. Design and features of BioMime™ Morph SES. SES: sirolimus-eluting stent. |
Procedure
PCI was performed according to the standard practices. The choice of access site, pre-dilatation or direct stenting, the use of glycoprotein IIb/IIIa inhibitors, deployment of a pressure wire, approach for treating branching or completely blocked artery segments, and stent selection including type, length, and diameter were at the operator’s discretion. A staged procedure was allowed if necessary, and such cases were not considered as instances of revascularization. During the index procedure, anticoagulation was maintained using unfractionated heparin. Following PCI, patients were prescribed antiplatelet therapy (aspirin + ticagrelor/clopidogrel/prasugrel) as per the investigator’s discretion and in accordance with the standard guidelines of the American College of Cardiology/American Heart Association/European Society of Cardiology (ACC/AHA/ESC). Procedural success, device success, freedom of target lesion failure (TLF), and target vessel failure (TVF) were recorded to evaluate the performance of BioMime™ Morph SES.
Endpoints and follow-up
Safety endpoints
The safety endpoints were major adverse cardiovascular events (MACEs), cardiac death, MI, ischemia-driven target lesion revascularization (ID-TLR), ischemia-driven target vessel revascularization (ID-TVR), and ST at each follow-up till 36 months.
MACE was defined as a composite of cardiac death, MI attributed to the target vessel or ID-TLR. Cardiac death was defined as any death resulting from an acute MI, sudden cardiac death, death due to heart failure, or death due to stroke. MI was defined as the development of new, pathological Q waves on electrocardiogram, or elevation of creatinine kinase (CK) ≥ 2-fold the upper limit of normal with elevated CK-MB in the absence of new pathological Q waves. ID-TLR included any repeat PCI of the target lesion or bypass surgery of the target vessel performed for restenosis or other complications of the target lesion. ID-TVR included any repeat percutaneous intervention or surgical bypass for any segment in the target vessel. ST was defined as the presence of a thrombus that originates in the stent or in the segment of 5 mm proximal or distal to the stent and the presence of at least one of the following criteria within a 48-h time frame: 1) acute onset of ischemic symptoms at rest; 2) new ischemic electrocardiogram (ECG) changes that suggest acute ischemia; and 3) typical rise and fall in cardiac biomarkers.
Performance endpoints
The performance endpoints were TVF and freedom from TLF at each follow-up till 36 months. Additional performance endpoints included procedural success defined as angiographic evidence of < 30% final residual stenosis of the target lesion after stent placement and no occurrence of a procedure-related MACE prior to hospital discharge (subjects implanted with stents for more than 1 lesion, the worse outcome was considered) and device success defined as angiographic evidence of < 30% final residual stenosis of the target lesion using only the assigned device. Freedom from TLF was defined as a composite of cardiac death, MI attributed to the target vessel, and TLR. TVF was defined as the composite of cardiac death, MI attributed to the target vessel, or TVR.
The patients were followed up at 1, 6, 12, 24, and 36 months post-procedure. During the follow-up, the occurrence of MACE, cardiac death, MI, ID-TLR, ID-TVR, and ST was recorded.
Statistical analysis
The data were analyzed based on the assessment of outcome measures for the study population on an intention-to-treat basis. Continuous variables are summarized as means ± standard deviations, while categorical data are presented as numbers/frequency with percentages. The safety analysis was performed after excluding the dropouts and subjects lost to follow-up. The statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) version 21 (IBM Corporation, Chicago, IL, USA). The Kaplan-Meier estimate was used for the presentation of MACEs.
| Results | ▴Top |
A total of 88 patients were enrolled in the study. All subjects completed the 3-year follow-up after the index procedure (Fig. 2).
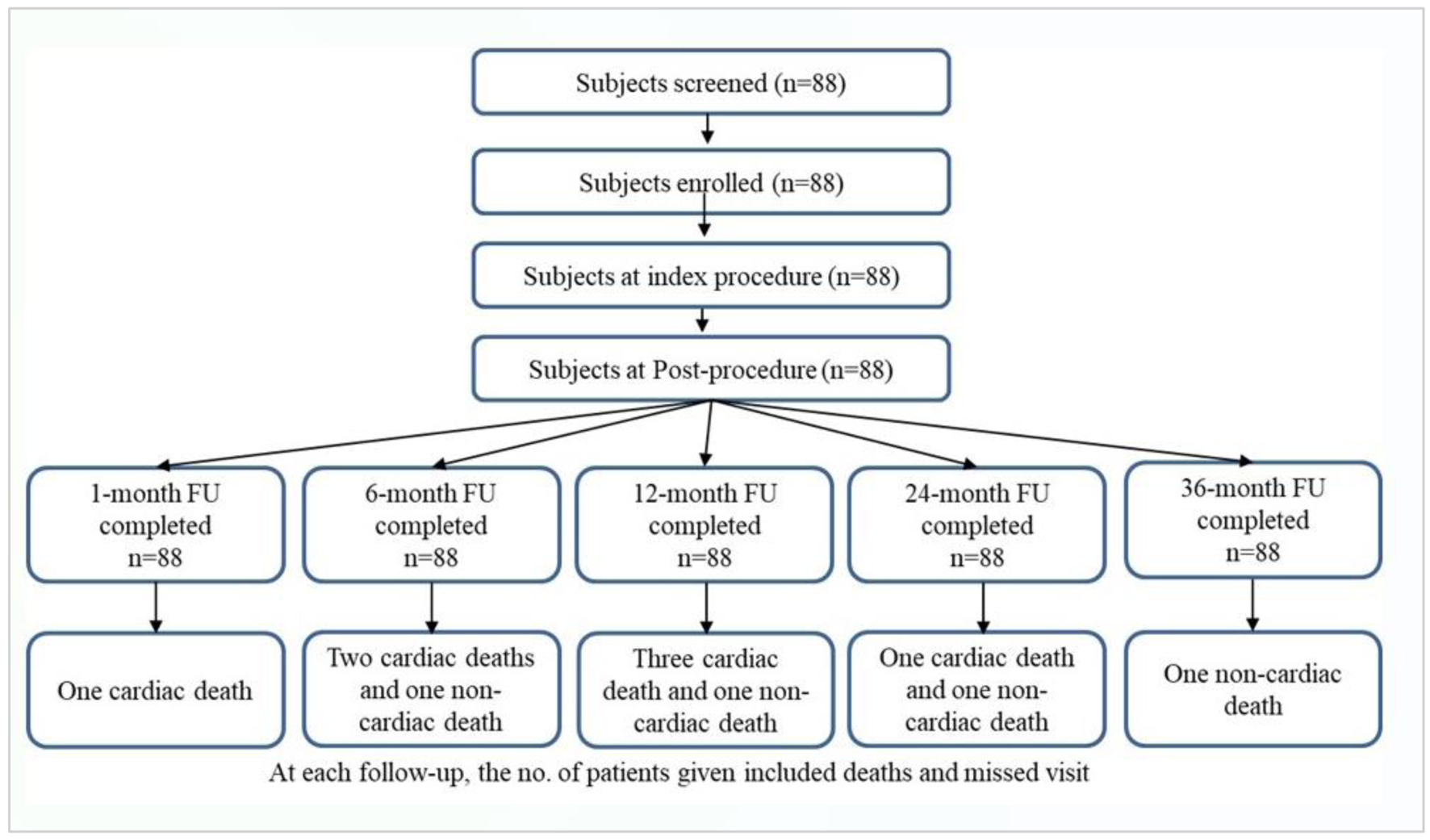 Click for large image | Figure 2. Study flowchart of patient enrolment and follow-up. |
Baseline and demographic characteristics
The eligible study population comprised 82.95% men and 17.05% women, with a mean age of 58.72 ± 10.10 years. Most patients had a history of angina (81.82%) and ischemic heart disease (IHD) (78.41%). The leading risk factors for cardiovascular disease including diabetes mellitus (59.09%) and hypertension (54.55%) were also prevalent in our study population. The mean left ventricular ejection fraction (LVEF) at baseline was 50.23±9.22%. Almost 50% of patients presented with ST-elevation myocardial infarction (STEMI), followed by unstable angina in 12.50%. The baseline characteristics of the patients are shown in Table 1.
 Click to view | Table 1. Baseline and Demographic Characteristics |
Lesion characteristics
A total of 92 de novo coronary lesions with an average lesion length of 43.32 ± 9.46 mm and mean diameter stenosis of 90.17 ± 9.93% were implanted with of BioMime™ Morph SESs (1.05 stents/patient). The lesion characteristics are shown in Table 2.
 Click to view | Table 2. Lesion Characteristics |
All lesions were successfully implanted with a long, tapered BioMime™ Morph SES alone, with the device and procedural success rates of 100%. The average length of the implanted study device was 45.54 ± 10.20 mm and the average diameter was 2.99 ± 0.29 mm. The majority of the procedures were performed through a transfemoral approach (97.73%). The ionic contrast media were the most commonly used media in all procedures. In most patients, both pre-dilatations and post-dilatations were performed. The thrombolysis in myocardial infarction (TIMI) flow improved to grade III in all lesions, signifying the re-establishment of myocardial reperfusion. The procedural outcomes are shown in Table 3. All the patients were prescribed dual antiplatelet therapy: clopidogrel (n = 82), prasugrel (n = 1) and ticagrelor (n = 5). Antilipidemic drugs (atorvastatin (n = 6) and statin (n = 64)) were prescribed in 70 (79.54%) patients.
 Click to view | Table 3. Procedural Outcomes |
Outcomes at 3 years
All the patients were event-free during the hospital stay with the freedom from TLF rate of 100%. In terms of early events (1 month), one patient had MI, which led to TLF and ultimately resulted in cardiac death. At 1 month, no cases of non-cardiac death were reported and 98.86% of patients were free from TLF. During 3-year follow-up, the cumulative MACE rates were 7.95% including 7.95% of cardiac deaths due to MI. The MACE free survival rate was 92% at 36 months of follow-up as shown in Figure 3. No cases of ID-TLR, ID-TVR, and ST were reported till the final follow-up. The cumulative 3-year outcomes are shown in Table 4.
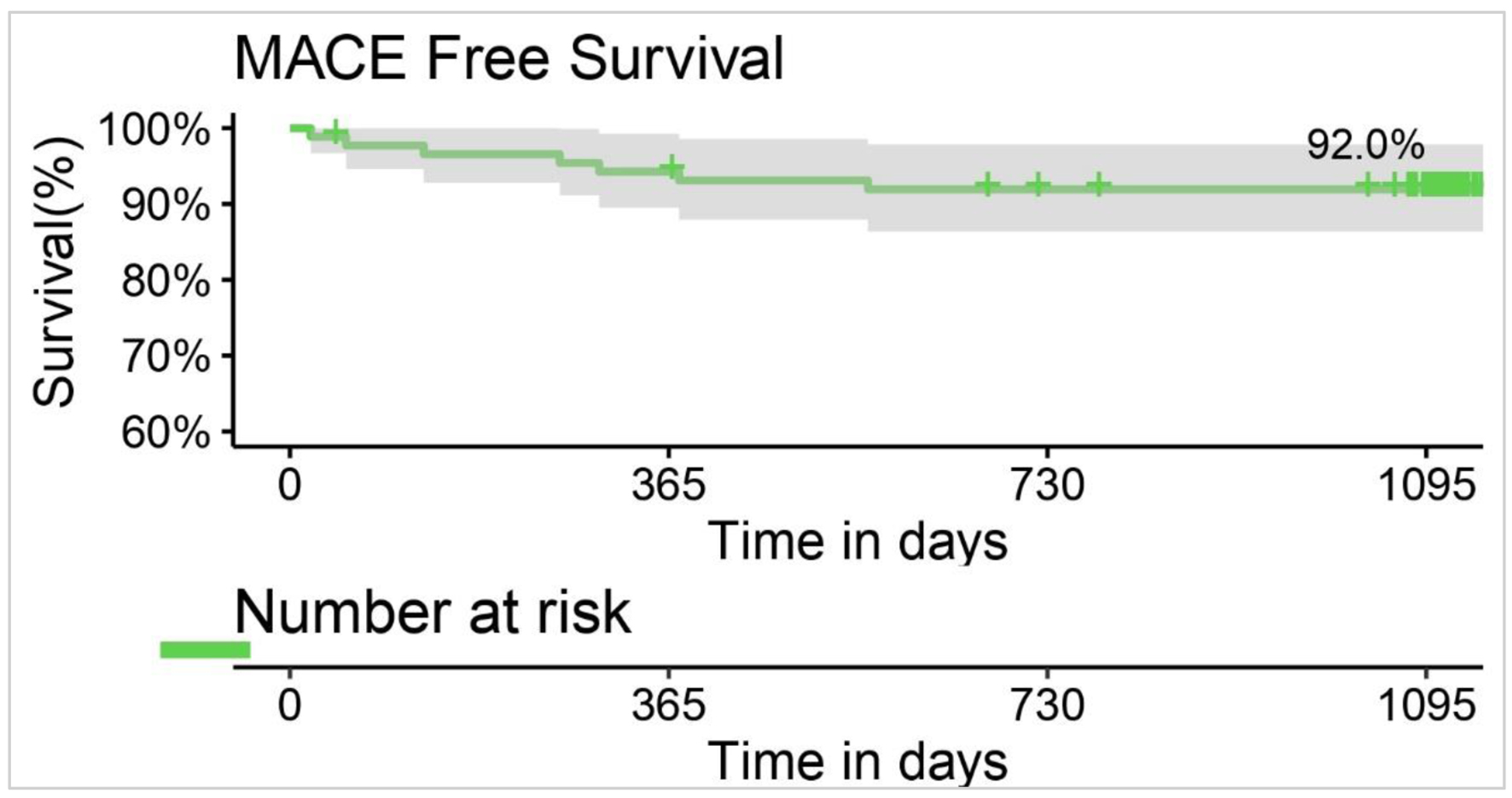 Click for large image | Figure 3. Kaplan-Meier curve for MACE free survival at 36 months. MACE: major adverse cardiovascular event. |
 Click to view | Table 4. Cumulative Clinical Events up to 36 Months Follow-Up |
| Discussion | ▴Top |
The results of our study suggest that the BioMime™ Morph SES has favorable outcomes up to 3 years in the treatment of very long lesions, evidenced by an acceptable rate of MACEs and absence of ST. The outcomes were favorable although more than 50% of the cohort had TIMI grades 0 and 1 before the procedure and 53.26% required stent sizes of 50 and 60 mm.
More than 20% of PCI cases in modern clinical practice are associated with diffuse long lesions, which are a significant contributor to adverse clinical outcomes [23]. The IRIS-DES registry that stratified patients with long lesions and/or small vessel diameters based on the cutoff points of the stent parameters, found that poor clinical outcomes were associated with a greater stent length and smaller stent diameter. This registry showed that a stent length of 43.0 mm was the differential cutoff for predicting the risk of TVF with second-generation DES [24]. Considering these factors, our outcomes are very encouraging.
In a single-center, non-randomized, comparative study by Im et al, the 3-year outcomes of two groups including patients with long lesions (> 30 mm) treated with zotarolimus-eluting stent and SES were compared. The results of the SES arm (n = 265) showed an ID-TLR rate of 4.6% and a 2.4% rate of definite ST, which were higher than those in our study. However, the cardiac death and MI rates in their study were lower due to a greater utilization rate of intravascular ultrasound (IVUS) (62% lesions in the SES group) leading to better stent expansion in complex lesions. Despite the lack of IVUS, our study showed 0% TLR and no ST events [25].
Kang et al previously compared the 12-month outcomes of three randomized clinical trials of the LONG-DES (LONG-DES III, IV, and V trials) in percutaneous treatment of LONG native coronary lesions with DES. The comparison of their findings with those of our study is shown in Table 5 [23, 26-32]. Our MACE outcomes are superior to all studies compared by Kang et al [23]. Importantly, despite the average stent length used in our study being 45.54 ± 10.20 mm, there were no cases of ST, ID-TLR, and ID-TVR up to 3 years of follow-up. Among other studies on long stents, the SPIRIT 48 trial evaluated the safety and efficacy of the XIENCE Skypoint 48 mm DES in 107 subjects with coronary artery disease having long de novo native coronary lesions. Though the device success rate was 97.2%, the rate of cardiac death/all MI at 1 year was 5.8% and the TLF rate was 5.7%, which were much higher than the rates with BioMime™ Morph SES [33].
 Click to view | Table 5. Comparison of Clinical Outcomes Involving Contemporary DES in Long Lesion Trials |
Previous studies have reported outcomes with BioMime™ Morph SES in long coronary lesions. Among the initial studies was one by Matchin et al, who reported a 98.8% clinical success rate. Restenosis was seen in 10.4% of patients at 12 months [15]. Subsequently, Patted et al reported a cumulative MACE incidence of 2.0% and ST of 0.3% at 12-month follow-up with the same stent [14]. Podolec et al reported an MACE rate of 0% at 3, 6, and 12 months in a small cohort of 32 patients [16]. Jurado-Roman et al reported an MACE rate of 6.2% and an ST rate of 0% at 20 months [18], while Sharma et al reported a 1-year MACE rate of 4.7% [21]. We compared our results with some additional studies on long coronary lesions as shown in Table 5. The incidences of ST were 0% in our study, which was noted in very few studies like Karmpaliotis et al [28] and PtCr-EES, Re-ZES groups of Kang et al [23]. It is also worth noting that in almost all previous publications, the TLR and TVR rates were higher than our study (0%).
All previous studies of BioMime™ Morph SES reported only immediate post-procedure or 1-year outcomes, except one that reported 20-month outcomes. Ours is the first study to report 3-year outcomes with the BioMime™ Morph SES in real-world settings.
Nevertheless, there are some limitations to our study. The first is the lack of a comparator arm with another long stent or multiple stents. Further, the study lacks follow-up angiographic data; hence, we could not confirm the outcomes objectively. Further long-term studies with comparator arms comprising multiple stents for long lesions are necessary to validate these outcomes. The relatively small sample size might have limited the ability to assess rare clinical adverse outcomes.
Conclusion
The BioMime™ Morph SES appears to have favorable outcomes up to 3 years in the treatment of very long coronary lesions in native coronary arteries, as demonstrated by an acceptable rate of MACEs and absence of stent thrombosis, over 3 years.
Acknowledgments
Writing support for the manuscript was provided by Dr. Sangeeta Dhanuka.
Financial Disclosure
This work was supported by Meril Life Sciences Pvt. Ltd, India.
Conflict of Interest
None to declare.
Informed Consent
Written informed consent was obtained from all subjects before enrolment.
Author Contributions
Suresh Patted: conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; visualization.
Abbreviations
CK: creatinine kinase; DES: drug-eluting stent; ID-TLR: ischemia-driven target lesion revascularization; ID-TVR: ischemia-driven target vessel revascularization; IVUS: intravascular ultrasound; LAD: left anterior descending; LCx: left circumflex; PCI: percutaneous coronary intervention; PLGA: poly (D,L-lactide-co-glycoside); MACE: major adverse cardiovascular event; MI: myocardial infarction; PLLA: poly (L-lactide); PTCA: percutaneous transluminal coronary angioplasty; RCA: right coronary artery; SES: sirolimus-eluting stent; ST: stent thrombosis; TLF: target lesion failure; TVF: target vessel failure
| References | ▴Top |
- Premchand RK, Kumar YS. A report of successful procedural, clinical, and angiographic outcomes with a tapered stent of a patient in naturally tapered coronary vessel. J Clin Diagn Res. 2017;11(1):OD06-OD07.
doi pubmed pmc - Roach MR, MacLean NF. The importance of taper proximal and distal to Y-bifurcations in arteries. Front Med Biol Eng. 1993;5(2):127-133.
pubmed - Banka VS, Baker HA, 3rd, Vemuri DN, Voci G, Maniet AR. Effectiveness of decremental diameter balloon catheters (tapered balloon). Am J Cardiol. 1992;69(3):188-193.
doi pubmed - Zhang LR, Xu DS, Liu XC, Wu XS, Ying YN, Dong Z, Sun FW, et al. [Coronary artery lumen diameter and bifurcation angle derived from CT coronary angiographic image in healthy people]. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39(12):1117-1123.
pubmed - Zubaid M, Buller C, Mancini GB. Normal angiographic tapering of the coronary arteries. Can J Cardiol. 2002;18(9):973-980.
pubmed - Kozlik M, Harpula J, Chuchra PJ, Nowak M, Wojakowski W, Gasior P. Drug-eluting stents: technical and clinical progress. Biomimetics (Basel). 2023;8(1):72.
doi pubmed pmc - Ng J, Foin N, Ang HY, Fam JM, Sen S, Nijjer S, Petraco R, et al. Over-expansion capacity and stent design model: An update with contemporary DES platforms. Int J Cardiol. 2016;221:171-179.
doi pubmed - Noad RL, Hanratty CG, Walsh SJ. Clinical impact of stent design. Interv Cardiol. 2014;9(2):89-93.
doi pubmed pmc - Cook S, Wenaweser P, Togni M, Billinger M, Morger C, Seiler C, Vogel R, et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation. 2007;115(18):2426-2434.
doi pubmed - Uren NG, Schwarzacher SP, Metz JA, Lee DP, Honda Y, Yeung AC, Fitzgerald PJ, et al. Predictors and outcomes of stent thrombosis: an intravascular ultrasound registry. Eur Heart J. 2002;23(2):124-132.
doi pubmed - Fujii K, Carlier SG, Mintz GS, Yang YM, Moussa I, Weisz G, Dangas G, et al. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J Am Coll Cardiol. 2005;45(7):995-998.
doi pubmed - Gasior P, Lu S, Ng CKJ, Toong WYD, Wong EHP, Foin N, Kedhi E, et al. Comparison of overexpansion capabilities and thrombogenicity at the side branch ostia after implantation of four different drug eluting stents. Sci Rep. 2020;10(1):20791.
doi pubmed pmc - Raber L, Juni P, Loffel L, Wandel S, Cook S, Wenaweser P, Togni M, et al. Impact of stent overlap on angiographic and long-term clinical outcome in patients undergoing drug-eluting stent implantation. J Am Coll Cardiol. 2010;55(12):1178-1188.
doi pubmed - Patted SV, Jain RK, Jiwani PA, Suryavanshi S, Raghu TR, Raveesh H, Rajalakshmi S, et al. Clinical outcomes of novel long-tapered sirolimus-eluting coronary stent system in real-world patients with long diffused de novo coronary lesions. Cardiol Res. 2018;9(6):350-357.
doi pubmed pmc - Matchin YG, Atanesyan RV, Kononets EN, Danilov NM, Bubnov DS, Ageev FT. [The first experience of using very long stents covered with sirolimus (4060 mm) in the treatment of patients with extensive and diffuse lesions of the coronary arteries]. Kardiologiia. 2017;57(4):19-26.
pubmed - Podolec J, Skubera M, Niewiara L, Podolec M, Pieniazek P, Bartus K, Zmudka K, et al. Clinical experience with 12-month follow-up in patients after implantation of a novel long-tapered sirolimus drug-eluting stent. Postepy Kardiol Interwencyjnej. 2019;15(1):46-51.
doi pubmed pmc - Lupi A, Ugo F, De Martino L, Infantino V, Iannaccone M, Iorio S, Di Leo A, et al. Real-world experience with a tapered biodegradable polymer-coated sirolimus-eluting stent in patients with long coronary artery stenoses. Cardiol Res. 2020;11(4):219-225.
doi pubmed pmc - Jurado-Roman A, Abellan-Huerta J, Requena JA, Sanchez-Perez I, Lopez-Lluva MT, Maseda-Uriza R, Piqueras-Flores J, et al. Comparison of clinical outcomes between very long stents and overlapping stents for the treatment of diffuse coronary disease in real clinical practice. Cardiovasc Revasc Med. 2019;20(8):681-686.
doi pubmed - Minana G, Consuegra-Sanchez L, Rumiz E, Valero E, Garcia-Blas S, Pernias V, Husser O, et al. Feasibility of implanting 50-60 mm-tapered drug eluting stents in chronic total occlusions. Cardiovasc Revasc Med. 2019;20(12):1117-1122.
doi pubmed - Raghu TR, Raj VAS, Kharge J, Setty HSN, Patil RS, Manjunath CN. Feasibility and outcomes of left main to branch vessel PCI with novel tapered coronary stent in a tertiary care centre: a real world experience. Cardiovasc Hematol Disord Drug Targets. 2021;21(2):128-135.
doi pubmed - Sharma YP, Uppal L, Panda P, Mohanty S, Kasinadhuni G, Krishnappa D, Mehrotra S, et al. One-year outcomes of novel BioMime Morph tapered stent in long and multiple coronary artery lesions. Anatol J Cardiol. 2021;25(12):896-901.
doi pubmed pmc - Thevan B, Abdulrahman A, Subbramaniyam S, Chachar TS, Yousif N, Noor HA, Amin H, et al. Real-world experience with a 60-mm-long stent in the setting of primary percutaneous coronary intervention. Heart Views. 2022;23(3):133-137.
doi pubmed pmc - Kang DY, Jang JS, Chang M, Lee CH, Lee PH, Ahn JM, Lee SW, et al. Comparison of different types of drug-eluting stents for de novo long coronary artery lesions. JACC Asia. 2022;2(4):446-456.
doi pubmed pmc - Lee CH, Kang DY, Han M, Hur SH, Rha SW, Her SH, Seung KB, et al. Differential cutoff points and clinical impact of stent parameters of various drug-eluting stents for predicting major adverse clinical events: An individual patient data pooled analysis of seven stent-specific registries and 17,068 patients. Int J Cardiol. 2019;282:17-23.
doi pubmed - Im E, Kim BK, Ko YG, Her AY, Choi HH, Shin DH, Kim JS, et al. Comparison of 3-year clinical outcomes between Resolute zotarolimus- and sirolimus-eluting stents for long coronary artery stenosis. J Interv Cardiol. 2013;26(4):378-383.
doi pubmed - Gautier A, Hovasse T, Arroyo D, Unterseeh T, Garot P, Champagne S, Neylon A, et al. Safety and efficacy of 48 mm Xience Xpedition everolimus-eluting stent for the treatment of long coronary lesions. Catheter Cardiovasc Interv. 2022;100(2):179-187.
doi pubmed - Hsiao FC, Tsai CT, Hsu LA, Tung YC, Yu FC, Lin CP, Chou SH, et al. Procedural and one-year clinical outcomes of long 48 mm Xience Xpedition everolimus-eluting stent in complex long diffuse coronary artery lesions. J Invasive Cardiol. 2022;34(2):E80-E86.
pubmed - Karmpaliotis D, Stoler R, Walsh S, El-Jack S, Potluri S, Moses J, Oldroyd K, et al. Safety and efficacy of everolimus-eluting bioabsorbable polymer-coated stent in patients with long coronary lesions: the EVOLVE 48 study. Catheter Cardiovasc Interv. 2022;99(2):373-380.
doi pubmed pmc - Sim HW, Thong EH, Loh PH, Lee CH, Chan MY, Low AF, Tay EL, et al. Treating very long coronary artery lesions in the contemporary drug-eluting-stent era: single long 48 mm stent versus two overlapping stents showed comparable clinical outcomes. Cardiovasc Revasc Med. 2020;21(9):1115-1118.
doi pubmed - Paszek E, Zajdel W, Musialek P, Sokolowski A, Guzik B, Kablak-Ziembicka A, Niewiara L, et al. Percutaneous management of long and diffused coronary lesions using newer generation drug-eluting stents in routine clinical practice: long-term outcomes and complication predictors. Pol Arch Intern Med. 2019;129(6):392-398.
doi pubmed - Diaz Fernandez JF, Camacho Freire SJ, Fernandez Guerrero JC, Delarche N, Bretelle C, Zueco Gil J, Palop RL, et al. Everolimus drug-eluting stent performance in patients with long coronary lesions: the multicenter Longprime registry. Catheter Cardiovasc Interv. 2018;92(7):E493-E501.
doi pubmed - Rajesh GN, Sulaiman S, Vellani H, Sajeev CG. One-year clinical outcome of percutaneous coronary intervention with very long (>/= 40mm) drug-eluting stent. Indian Heart J. 2018;70(Suppl 3):S285-S289.
doi pubmed pmc - Park KE, Wu CJ, Chehab B, Maksoud A, Bertolet B, Ying SW, Simoes T, et al. One-year outcomes of XIENCE Skypoint 48-mm drug-eluting stents in long coronary lesions: the SPIRIT 48 trial. J Soc Cardiovasc Angiogr Interv. 2023;2(4):101001.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


