| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 15, Number 5, October 2024, pages 396-403
Detection of Left Atrial Remodeling by Three-Dimensional Echocardiography in Symptomatic Patients Known to Had Non-Obstructive Hypertrophic Cardiomyopathy
Taher Said Abd Elkareema, b, e, Shaimaa Habibc, Amr Shehatad, Fatma Elhadyc
aDepartment of Cardiology, Nizwa Hospital, Nizwa, Oman
bDepartment of Cardiology, Islamic Center of Cardiology, Al Azhar University, Cairo, Egypt
cDepartment of Cardiology, Faculty of Medicine for Girls, Al Azhar University, Cairo, Egypt
dDepartment of Cardiology, Benha Teaching Hospital, General Organization for Teaching Hospitals and Institutes, Cairo, Egypt
eCorresponding Author: Taher Said Abd Elkareem, Islamic Center of Cardiology, Al Azhar University, Cairo, Egypt
Manuscript submitted June 22, 2024, accepted July 24, 2024, published online August 31, 2024
Short title: LA Remodeling by 3D Echo
doi: https://doi.org/10.14740/cr1690
| Abstract | ▴Top |
Background: Hypertrophic cardiomyopathy (HCM) is one of the most prevalent inherited disorders and a common cause of sudden heart death. Left atrial (LA) dilatation frequently occurs in patients with HCM as a result of impaired left ventricular (LV) relaxation or associated involvement of LA myocardium in HCM.
Methods: We enrolled 170 patients known to had HCM (non-obstructive type) and 30 healthy subjects (control group). All of them underwent two-dimensional (2D) echocardiography to measure LV dimensions, function, LA dimension, LA deformations, pulmonary artery pressure (PAP) and LV global longitudinal strain (LVGLS). LA volumes and mechanics were also measured by three-dimensional (3D) echocardiography.
Results: By 2D echocardiography, patient group revealed significantly lower all LA functions vs. control group including reservoir (26 ± 4 vs. 43 ± 3, P < 0.001), conduit (-14 ± 2 vs. -25 ± 2, P < 0.001), and booster pump functions (-12 ± 2 vs. -18 ± 1, P < 0.001). PAP was significantly higher in patient group (42 ± 7 vs. 27 ± 4 in control group). LVGLS was significantly lower in patient group (-15±1.4% vs. -23±2% in control group). Using 3D speckle tracking echocardiography (STE), there were a significantly higher indexed maximum LA volume (Vmax indexed) (43.5 ± 5.6 vs. 28.7 ± 3.7, P < 0.001), but significantly lower left atrial strain at reservoir function (LASr) (24 ± 4 vs. 41 ± 3, P < 0.001), left atrial strain at conduit function (LAScd) (-13 ± 2 vs. -24 ± 2, P < 0.001), and left atrial strain at contractile function (LASct) (-11 ± 2 vs. -18 ± 1, P < 0.001).
Conclusion: Three-dimensional transthoracic echocardiography (TTE) is a feasible method for the assessment of LA remodeling, but there is adverse LA remodeling in patients with long-standing non-obstructive HCM including impaired all LA mechanics and with increased septal thickness, there are more diastolic dysfunction and more reduction of LA mechanics.
Keywords: HCM; 3D TTE; LA strain; LA mechanics
| Introduction | ▴Top |
Hypertrophic cardiomyopathy (HCM) is one of the most prevalent inherited disorders and a common cause of sudden heart death with a frequency of up to one case per 500 populations. Aberrant left ventricular hypertrophy (LVH), which is caused by an aberrant myocardial fiber array, is the hallmark of HCM [1]. Diastolic dysfunction develops and myocardial relaxation is compromised as a result of LVH. The most prevalent arrhythmia linked to HCM is atrial fibrillation (AF), which is strongly correlated with both left atrial (LA) dilatation and elevated left ventricular (LV) pressure that reflected back and resulted in increased LA pressure [2].
There are three components of LA function: 1) Blood can be accumulated close to the closed mitral valve (MV) during (LV) systole because the LA functions as a reservoir during this time. 2) The LA has a conduit function during the early stages of LV diastole, which causes it to shorten. 3) The LA functions as a booster pump in late diastole [1].
Prior studies concluded that HCM is associated with impaired LA function [3].
Changes in the size or function of the LA have been linked to unfavorable cardiovascular outcomes because of its significant role. For instance, an increased risk of AF and stroke is linked to LA enlargement [4].
Three-dimensional (3D) echocardiography is more accurate than two-dimensional (2D) echocardiography in the assessment of LA volumes because it avoids errors like geometrical assumptions of LA shape and foreshortening in the 2D views [5]. The current study aimed at evaluating the impact of HCM on LA functions using 2D and 3D echocardiography, as well as the relationships between these functions and diastolic function, pulmonary artery pressure (PAP), and interventricular septum thickness (IVST).
| Materials and Methods | ▴Top |
Study design and populations
One hundred and seventy patients (patient group) and 30 healthy patients (control group) were included in the present study. All members of the control group had no chronic illness and their age was less than 40 years to eliminate any factors affecting LA functions. All patients were known to have non-obstructive HCM. Diagnosis was done either by genotyping, first-degree relative of HCM patients or by cardiac magnetic resonance (CMR) and all of them had asymmetrical septal wall hypertrophy, referred for follow-up echo-Doppler assessment. The study was conducted at the Islamic Center of Cardiology and Alzahraa Hospital, Al Azhar University, Egypt. This study was ethically approved by Al Azhar University’s Research Ethics Committee (2367/14-05-2024). The study protocol conformed to the Helsinki Declaration, the ethical norm of the World Medical Association for human testing. Data were collected between January 2022 and March 2024.
The current study excluded patients with poor echocardiography window and patients with known chronic illness that may affect LA functions (such as hypertension, diabetes), significant aortic stenosis (AS) or aortic regurgitation (AR), and mitral regurgitation (MR) or mitral stenosis (MS). Patients with ischemic, pericardial, or congenital heart disease as well as those with AF or frequent ectopics were also excluded.
All patients underwent complete history taking, clinical examination, and resting 12-lead electrocardiogram.
Transthoracic echo-Doppler scan
A 2.5 multi-frequency 1.7 - 4 MHz transducer (GE Vivid 95 Ultrasound Machine) was used for the examination, and GE Echo pack 204 software was used for the offline analysis of the 2D and 3D speckle tracking echocardiography (STE). The ensuing information was acquired: 1) Measurement of the following parameters using 2D-guided M-mode: left ventricular end-systolic dimension (LVESD) and left ventricular end-diastolic dimension (LVEDD) (mm2), left ventricular ejection fraction (LVEF, %), fractional shortening (%), end-diastolic diameter (mm) of the interventricular septum (IVSD), end-diastolic diameter (mm) of the LV posterior wall (LVPWDD), and LA dimension (mm). 2) Assessment of the E/A ratio and mitral E and A wave velocities (cm/s) using a conventional pulsed wave (PW) Doppler. 3) Assessment of MR’s degree and presence using a conventional continuous wave Doppler; estimation of systolic PAP (mm Hg) using the Bernoulli equation derived from the velocity of tricuspid regurgitation (TR). 4) Measurement of S wave velocity, Ea wave velocity, Aa wave velocity, and E/Ea ratio using tissue Doppler imaging.
Using 2D STE, apical LV three-chamber images, apical four-chamber, two-chamber were recorded at high frame rates (range: 59 - 82 frame/s; mean 72 + 6 frame/s) in order to quantify LV longitudinal strain. Three successive cardiac cycles were recorded in each plane while the patient was on breath hold, and the data were digitally saved on a hard drive for later examination. PW Doppler echocardiography was used to record the LV input and outflow velocities to record the cardiac events timing.
Furthermore, we quantified LA strain using 2D STE. Using high frame rate acquisitions of apical four-chamber and two-chamber data (range: 59 - 82 frame/s; mean 72 + 6 frame/s), we determined planar LA strain in the reservoir, conduit, and contraction stages.
With 3D transthoracic echocardiography (TTE), an apical four-chamber image was captured at high frame rates (up to more than 25 frames per second), using a multi-beat modality to increase the frame rate as the patient was exhaling, and the image was digitally stored on a hard drive for offline use. In the reservoir, conduit, and contraction function, we quantified the left atrium ejection fraction (LAEF), LA strain, and LV volumes (maximum, indexed, minimum, and pre-A LA volumes).
Statistical methods
IBM (SPSS version 28, Armonk, New York, USA) was used for data administration and statistical analysis. Using direct data visualization techniques and the Shapiro-Wilk test, quantitative data were evaluated for normalcy. The means and standard deviations were used to summarize the quantitative data. Numbers and percentages were used to summarize the categorical data. The independent t-test was utilized to compare quantitative data among the groups under investigation. To compare categorical data, the Chi-square test was employed. Pearson’s correlation was used for correlation analyses. Each and every statistical test has two sides. Significant P values were those with a value of less than 0.05.
| Results | ▴Top |
General characteristics
As shown in Table 1, age was significantly higher in the patient group (35 ± 10 years) than in the controls (28 ± 5) (P < 0.001). In patient group, 90 were males (52.9%) and 80 were female (47.05%). One hundred and forty patients had New York Heart Association (NYHA) I and 30 patients had NYHA II. In both groups, there were no significant differences regarding sex (P = 0.782), body mass index (BMI) (P = 0.201), and body surface area (BSA) (P = 0.910), as shown in Table 1.
 Click to view | Table 1. General Characteristics of Studied Groups |
2D echo parameters
The mean LVEDD and LVESD were 45 ± 4 and 27 ± 5, respectively. The mean IVSD and interventricular septal diameter in systole (IVSS) were 33 ± 7 and 36 ± 6, respectively. The mean left ventricular posterior wall diameter in diastole (LVPWDD) and left ventricular posterior wall diameter in systole (LVPWDS) were 13 ± 1 and 10 ± 1, respectively. Regarding aortic root dimension (AORD) and LA dimension, the mean values were 26 ± 3 and 43 ± 4, respectively. Regarding pressure gradient (PG), the mean was 10 ± 1 (resting) and 13 ± 2 (with Valsalva) as shown in Table 2.
 Click to view | Table 2. 2D Echo Doppler Dimensions of Studied Cases |
PAP and EF
The patient group demonstrated significantly higher PAP (42 ± 7 mm Hg) than controls (27 ± 4 mm Hg). In contrast, no significant difference in EF was detected (P = 0.223) as shown in Table 3.
 Click to view | Table 3. PAP and EF in Studied Groups |
LA mechanics by 3D echo (volumetric and strain indices)
There were significantly higher maximum LA volume (Vmax) (80 ± 9 vs. 50 ± 5, P < 0.001), indexed maximum LA volume (Vmax indexed) (43.5 ± 5.6 vs. 28.7 ± 3.7, P < 0.001), minimum LA volume (Vmin) (56 ± 10 vs. 30 ± 4, P < 0.001), and pre-LA contraction volume (Vpre-A) (69 ± 10 vs. 41 ± 6, P < 0.001). In contrast, there were significantly lower total LAEF (33 ± 5 vs. 45 ± 4, P < 0.001), left atrial strain at reservoir function (LASr) (24 ± 4 vs. 41 ± 3, P < 0.001), left atrial strain at conduit function (LAScd) (-13 ± 2 vs. -24 ± 2, P < 0.001), and left atrial strain at contractile function (LASct) (-11 ± 2 vs. -18 ± 1, P < 0.001) as shown in Table 4 and Figure 1.
 Click to view | Table 4. 3D LA Volumetric Indices of Studied Groups |
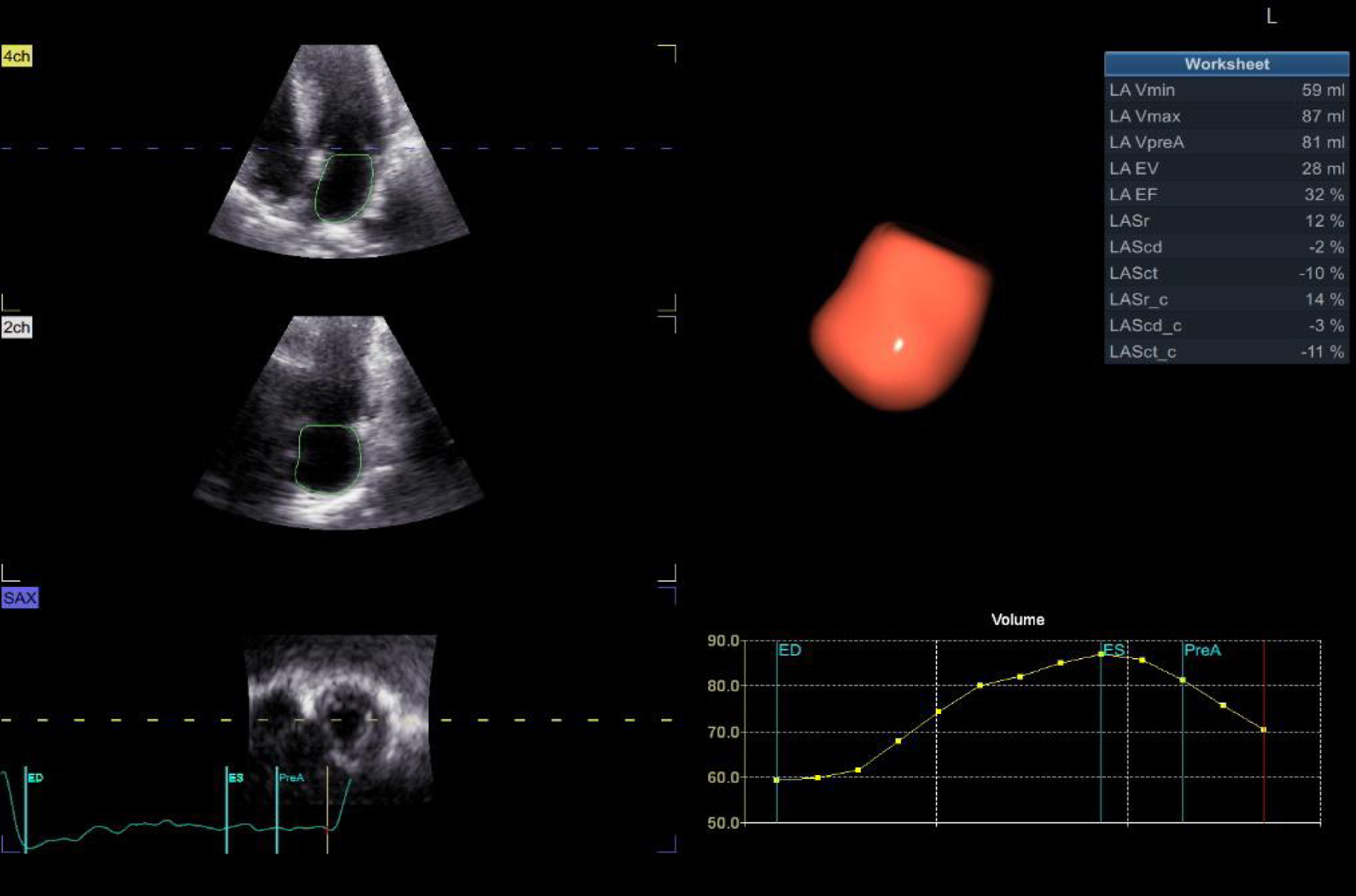 Click for large image | Figure 1. LA mechanics (volumetric and strain indices) by 3D echo. 3D: three-dimensional; LA: left atrial. |
PW transmitral inflow and tissue Doppler E/e
The peak A wave was significantly lower in patient group (0.58 ± 0.09) than in controls (0.94 ± 0.11) (P < 0.001). In contrast, the patients had a significantly higher E/e ratio (17.3 ± 4.3 vs. 8 ± 1, P < 0.001) as shown in Table 5.
 Click to view | Table 5. PW Transmitral Inflow and Tissue Doppler E/e’ in Studied Groups |
2D STE
The patient group demonstrated significantly lower LV global longitudinal strain (LVGLS) (-15 ± 1.4 vs. 22.5 ± 2, P < 0.001), peak LA strain at reservoir function (pLASRr) (26 ± 4 vs. 43 ± 3, P < 0.001), peak LA strain at conduit function (pLASRcd) (-14 ± 2 vs. -25 ± 2, P < 0.001), and peak LA strain at contractile function (pLASRct) (-12 ± 2 vs. -18 ± 1, P < 0.001) as shown in Table 6 and Figures 2 and 3.
 Click to view | Table 6. Speckle Tracking Parameters in Studied Groups |
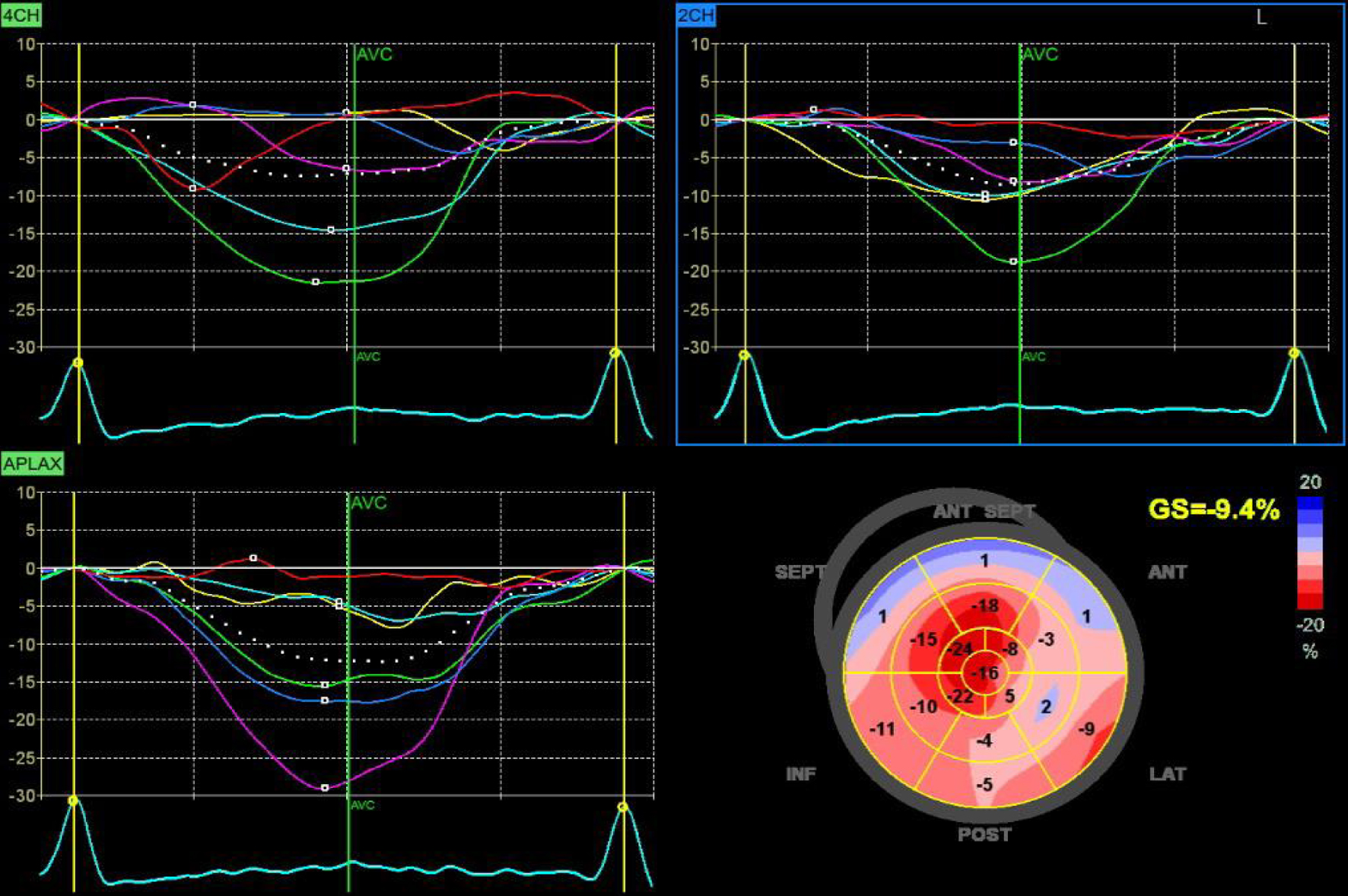 Click for large image | Figure 2. Global longitudinal strain by 2D speckle tracking echo. 2D: two-dimensional. |
 Click for large image | Figure 3. LA peak strain at reservoir, conduit and contractile functions by 2D speckle tracking echo. LA: left atrial. |
Correlation between IVSD and other parameters of patient group
IVSD showed significant positive correlations with E/e ratio (r = 0.513, P < 0.001), PAP (r = 0.903, P < 0.001), LVGLS (r = 0.754, P < 0.001), Vmax indexed (r = 0.806, P < 0.001), and Vmax (r = 0.859, P < 0.001) as shown in Table 7 and Figure 4.
 Click to view | Table 7. Correlation Between IVSD and Other Parameters |
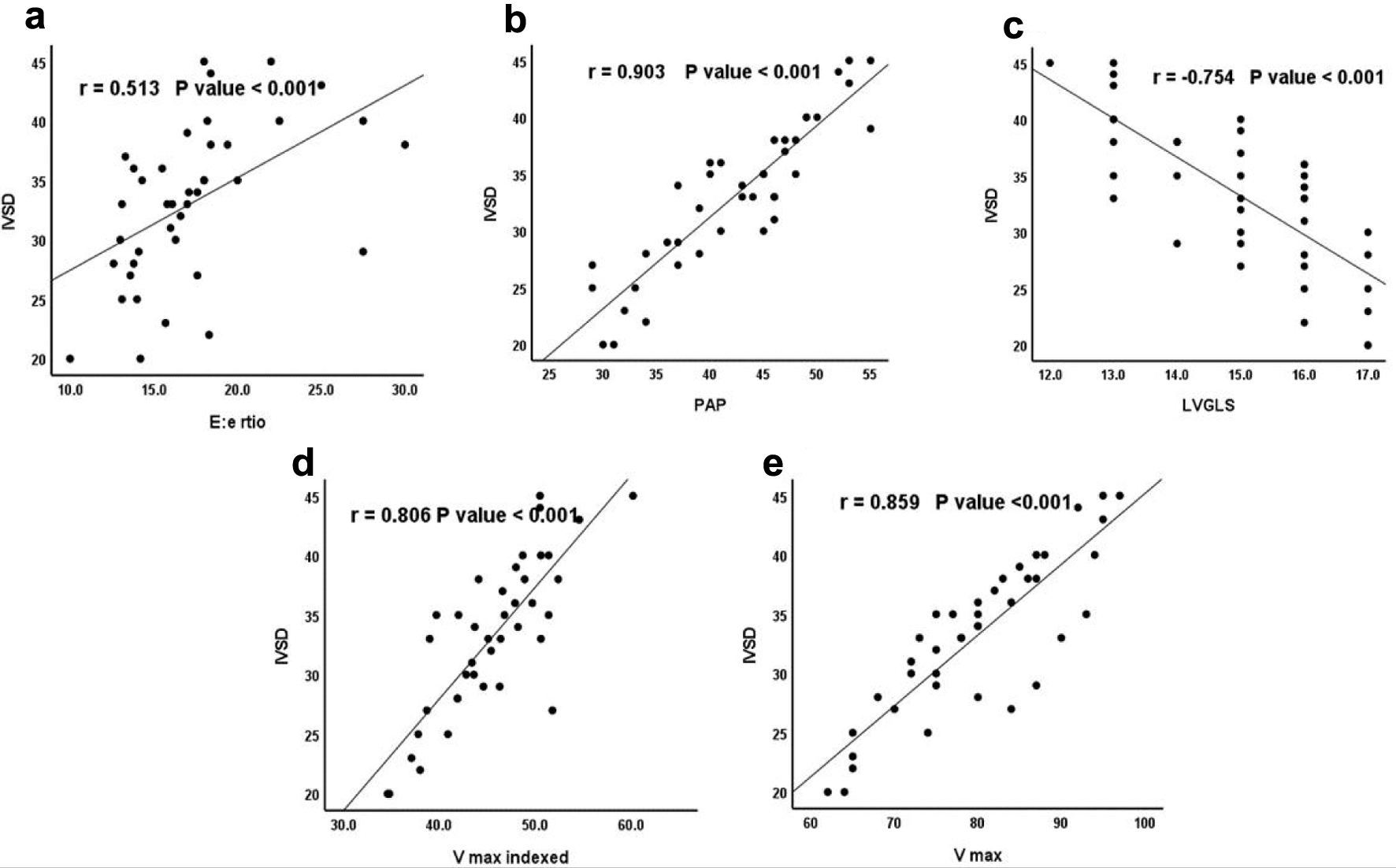 Click for large image | Figure 4. Correlation between IVSD and (a) E/e, (b) PAP, (c) LVGLS, (d) Vmax indexed, and (e) Vmax. IVSD: interventricular septal dimeter in diastole; LVGLS: LV global longitudinal strain; PAP: pulmonary artery pressure; Vmax: maximum LA volume; Vmax indexed: indexed maximum LA volume. |
| Discussion | ▴Top |
By acting as a conduit and promoting LV filling through atrial contraction, the LA is a crucial tissue that sits between the LV and the pulmonary circulation. Additionally, it protects the pulmonary system against recurrent oscillations of LV pressure, which occur when MR occurs.
There are three components of LA function: 1) Blood can be accumulated close to the closed MV during (LV) systole because the LA functions as a reservoir during this time. 2) The LA has a conduit function during the early stages of LV diastole, which causes it to shorten. 3) The LA functions as a booster pump in late diastole [1].
LA remodeling, demonstrated by increased LA size, impaired LA contractility, and interstitial fibrosis causing stiffness and reduced compliance results in increasing incidence of AF (up to four times in HCM patients more than general population) and also contributes to the development of post capillaries pulmonary hypertension related to left side heart disease. A combination of post- and pre-capillary pulmonary hypertension (CpcPH) results from pulmonary vasculature abnormalities such as intimal fibrosis and medial hypertrophy resulting in reduced vasodilator response, pulmonary vasoconstriction, and elevated pulmonary vascular resistance (PVR) with subsequent persistent changes in LA pressure [6, 7].
According to current standards, 3D TTE LA volume is the suggested parameter. The linear antero-posterior LA measurement, which can be derived from the parasternal long-axis view using M-mode or 2D TTE, has been the most often reported metric for years. This is because, when the LA enlarges, the antero-posterior diameter expects that all of its dimensions change in a similar way. However, this is frequently not the case during LA remodeling, particularly when the growth is eccentric. As a result, the LA size should not be determined exclusively by the antero-posterior linear dimension [5].
2D echocardiography and, more accurately 3D echocardiography, have gained large popularity as noninvasive modality for assessments of LA size and mechanical function. Since adverse LA remodeling is linked to an increased incidence of AF and cardiovascular adverse events, as demonstrated by Yang et al’s analysis of 104 HCM patients reporting serious cardiovascular complications and LA enlargement (LAVImax > 34 mL/m2) as well as greater LVH, and more diastolic dysfunction [8].
Regarding LA volumes measured by 3D TTE, the present study showed increased indexed LA maximum volume, pre-A LA volume (pre-LA contraction), and LA minimum volume compared to control group, and also there were decreased LA active emptying fraction and EF% compared to control group. These results were similar to those concluded by Shin et al, in spite of the small number of his study group [9]. But these results were discordant to those concluded by Kim et al, which showed increased indexed LA maximum volume and no statistically difference in LA active emptying fraction between patient and control groups [10]. This may be because they used 2D TTE for evaluation of LA volumes which is less accurate than 3D TTE. They also showed older age in their study group than the present study, which (older age) per say may affect the LA dimension and volumes.
LV filling pressures, LV elastic recoil, LVGLS, and LA pumping function are some of the factors that affect different components of LA functions. For example, the LA reservoir function measures the expansion of LA during systole while accounting for the initial volume of the atrium at the beginning of systole. The LA contractile function is directly reliant on the LA systolic function and is influenced by both LA systolic function and LA afterload. The LA conduit function measures the change in LA volume during early diastole and is influenced by LV early diastolic recoil as indicated by mitral annulus e’ velocity [11].
The present study showed reduced LVGLS either by 2D TTE or 3D TTE, concordant with the results of Shin et al [9] and Kim et al [10].
By using 3D STE, the present study showed marked reduction in the LA strain during reservoir function and this is because in HCM there are marked reduction in LV elastic recoil and reduction in LVGLS and this result was concordant with that concluded by Yang et al [3], which showed marked reduction in LA strain during reservoir function of LA in non-obstructive HCM. Also there was a marked reduction in LA strain during conduit function in the present study and this is due to marked reduction of mitral annular velocity in HCM and this result was similar to that conducted by Yang et al [3].
Also in our study, there was a marked reduction in LA strain during contractile function in patient group in comparison to control group due to increased LA afterload in HCM patients, concordant with that conducted by Shin et al [9] and discordant with that conducted by Yang et al [3] and Kim et al [10] that showed booster pump function of LA in the non-obstructive HCM patients was not significantly different than normal controls, and this may be due to the difference in the patient group between our study and their studies. Patient group in our study had more septal thickness and higher LV mass index (LVMI), and all of these parameters indicate higher LA afterload than their patient population that leads to impaired pump function after time. Another explanation to the impaired LA strain during LA contraction is direct involvement of atrial cardiomyocytes in sarcomeric disease and this result was similar to that conducted by Ramos et al [12] that concluded impaired LA strain in all components of LA function including contractile function in HCM patients more than hypertensive patients even in absence of diastolic function or LA dilatation.
There was a positive correlation between interventricular septal wall thickness and the ratio between peak MV inflow velocity/MV annular velocity (E/e’ ratio), and this is due to more septal thickness associated with marked reduction of MV annular motion and less LV elastic recoil. This results in more diastolic dysfunction, more increase in LA pressure and more congestive symptoms and this explains that most patients in the study group had NYHA III despite of apparent normal LVEF and no significant MR, concordant with that concluded by Aggul et al [13] that showed positive correlations between the development of pulmonary edema in HCM with reduced LA reservoir strain and increased LVMI.
Also there was a positive correlation between interventricular septal wall thickness and the development of pulmonary hypertension and this is due to the increase in LA pressure due to development of diastolic dysfunction plus marked impairment of LA reservoir, conduit and pump functions reflected to increase pulmonary venous congestion and development of post capillary pulmonary hypertension.
Conclusion
3D TTE is a feasible method for the assessment of LA remodeling. There are adverse LA remodeling in patients with long-standing non-obstructive HCM including impaired all LA mechanics and with increased septal thickness, there are more diastolic dysfunction and more reduction of LA mechanics.
Limitation
Despite the feasibility of 3D TTE in the assessment of LA remodeling, absence of the gold standard method for the assessment of LA remodeling (CMR) is one of the major limitations in this study. Another recommendation in future researches is to detect the incidence and the correlations of AF and the severity of LA dysfunction.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Written informed consents were obtained from all the participants.
Author Contributions
Taher Said Abd Elkareem did the transthoracic echo, did the analysis of TTE data in the vendor software, wrote the paper, and wrote the revised manuscript. Shaimaa Habib did the TEE to exclude contraindications for BMV, collected the data and inserted them in the excel sheet, helped the corresponding author to write the discussion. Amr Shehata helped in data collection, reviewed the grammar, also helped in discussion writing, collection of references, and sent the collected data for statistical analysis. Fatma Elhady reviewed the grammar, also helped in discussion writing, and collection of references.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Candan O, Gecmen C, Kahyaoglu M, Simsek Z, Celik M, Uslu A, Kirma C. Clinical utility of left atrial asynchrony and mechanical function in patients with hypertrophic cardiomyopathy. Acta Cardiol Sin. 2022;38(2):141-150.
doi pubmed pmc - Yang WI, Shim CY, Kim YJ, Kim SA, Rhee SJ, Choi EY, Choi D, et al. Left atrial volume index: a predictor of adverse outcome in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2009;22(12):1338-1343.
doi pubmed - Yang Y, Yin G, Jiang Y, Song L, Zhao S, Lu M. Quantification of left atrial function in patients with non-obstructive hypertrophic cardiomyopathy by cardiovascular magnetic resonance feature tracking imaging: a feasibility and reproducibility study. J Cardiovasc Magn Reson. 2020;22(1):1.
doi pubmed pmc - Her AY, Choi EY, Shim CY, Song BW, Lee S, Ha JW, Rim SJ, et al. Prediction of left atrial fibrosis with speckle tracking echocardiography in mitral valve disease: a comparative study with histopathology. Korean Circ J. 2012;42(5):311-318.
doi pubmed pmc - Badano LP, Miglioranza MH, Mihaila S, Peluso D, Xhaxho J, Marra MP, Cucchini U, et al. Left atrial volumes and function by three-dimensional echocardiography: reference values, accuracy, reproducibility, and comparison with two-dimensional echocardiographic measurements. Circ Cardiovasc Imaging. 2016;9(7):e004229.
doi pubmed - Abd Elkareem TS, Ahmed TA, Mohamed LA. Left atrial remodeling in patients with severe rheumatic mitral stenosis and sinus rhythm using two-dimensional and three-dimensional speckle tracking echocardiography. Cardiol Res. 2023;14(2):142-148.
doi pubmed pmc - Guazzi M, Naeije R. Pulmonary hypertension in heart failure: pathophysiology, pathobiology, and emerging clinical perspectives. J Am Coll Cardiol. 2017;69(13):1718-1734.
doi pubmed - Yang H, Woo A, Monakier D, Jamorski M, Fedwick K, Wigle ED, Rakowski H. Enlarged left atrial volume in hypertrophic cardiomyopathy: a marker for disease severity. J Am Soc Echocardiogr. 2005;18(10):1074-1082.
doi pubmed - Shin MS, Fukuda S, Song JM, Tran H, Oryszak S, Thomas JD, Shiota T. Relationship between left atrial and left ventricular function in hypertrophic cardiomyopathy: a real-time 3-dimensional echocardiographic study. J Am Soc Echocardiogr. 2006;19(6):796-801.
doi pubmed - Kim KJ, Choi HM, Yoon YE, Kim HL, Lee SP, Kim HK, Kim YJ, et al. Left atrial mechanical function and global strain in hypertrophic cardiomyopathy. PLoS One. 2016;11(6):e0157433.
doi pubmed pmc - Tayal B, Malahfji M, Buergler JM, Shah DJ, Nagueh SF. Hemodynamic determinants of left atrial strain in patients with hypertrophic cardiomyopathy: A combined echocardiography and CMR study. PLoS One. 2021;16(2):e0245934.
doi pubmed pmc - Ramos M, Loncaric F, Lopez A, et al. Differential left atrial function in hypertensive and sarcomeric left ventricular hypertrophy. European Heart Journal - Cardiovascular Imaging. 2023;24:jead119.332.
- Aggul B, Korkmaz B, Vatanoglu E, et al. Relationship between pulmonary edema development and left atrium mechanical functions in patients with left ventricular hypertrophy. European Heart Journal. 2020;41(Suppl 2):ehaa946.0085.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


