| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website http://www.cardiologyres.org |
Original Article
Volume 7, Number 2, April 2016, pages 51-58
Measuring B-Type Natriuretic Peptide From Capillary Blood or Venous Sample: Is It the Same?
Renato De Vecchisa, b, Carmelina Arianoa
aCardiology Unit, Presidio Sanitario Intermedio “Elena d’Aosta”, ASL Napoli 1 Centro, Napoli, Italy
bCorresponding Author: Renato De Vecchis, Cardiology Unit, Presidio Sanitario Intermedio “Elena d’Aosta”, ASL Napoli 1 Centro, via Cagnazzi 29, 80137 Napoli, Italy (Personal postal address: via P. Gaurico 21, 80125 Napoli, Italy)
Manuscript accepted for publication April 15, 2016
Short title: A New Point-of-Care Method for BNP
doi: http://dx.doi.org/10.14740/cr468e
| Abstract | ▴Top |
Background: In recent years, several systems have been implemented to achieve quick and non-invasive measurements of B-type natriuretic peptide (BNP). Among them, AlereTM Heart Check (AHC) BNP test represents the most recent advancement. It is a rapid point-of-care (POC) immunoassay, projected for measuring BNP directly from a capillary whole blood sample. This study aimed at comparing the analytical and clinical performances of this new POC to our reference method (Abbott Architect System).
Methods: One hundred eleven patients with stable chronic heart failure (CHF) referring to one cardiac rehabilitation center were enrolled from December 2013 to May 2015. These patients were subjected to a simultaneous capillary (AHC) and plasma (Abbott) BNP measurements. Clinical and analytical performances of AHC were assessed and compared to the reference method.
Results: Capillary BNP showed a good correlation with the reference method (r = 0.94, P < 0.0001), although the values diverged when BNP was higher than 1,500 pg/mL. Indeed, the AHC had a relatively poor precision and the coefficient of variability was 10.1% and 18% for low and high controls, respectively. However, both methods showed similar diagnostic performances in discriminating the patients with heart failure in New York Heart Association (NYHA) class I from those belonging to NYHA classes II-III, with values of area under the curve (AUC) of 0.983 and 0.984, respectively, and equivalent sensitivity, specificity, and positive and negative likelihood ratios.
Conclusion: The AHC BNP test is a good POC able to provide reliable information about the hemodynamic status of CHF patients, especially of those belonging to NYHA classes I-III.
Keywords: B-type natriuretic peptide; Point-of-care; Immunoassay; Heart failure; Capillary blood; Venous blood
| Introduction | ▴Top |
The B-type natriuretic peptide (BNP) and the amino-terminal fragment of the pro-B-type natriuretic peptide, known as NT-proBNP, are currently seen as gold standard biomarkers for the diagnosis and prognostic stratification of heart failure, according to international guidelines [1, 2]. These biomarkers are not only able to discriminate the origin of dyspnea (cardiac versus non-cardiac dyspnea, e.g. bronchial asthma) in the setting of emergency medicine [1, 2], but they are also useful for better managing patients with stable chronic heart failure (CHF). Indeed, in the last decade, there have been developments in the concept of BNP-guided therapy, in which the serial measurements of BNP over time are used to assess the effectiveness of drug treatment for heart failure, and to guide possible adjustments and modifications to dosage. However, the current methods for the measurement of BNP are invasive, requiring a venous blood sample (e.g. from the antecubital vein of the arm), with specific pre-analytical requirements in order to make the interpretation of the results feasible. In this context, the relative discomfort resulting from venipuncture and the relatively long period of time needed to get the test results could be an obstacle to a strict careful monitoring of the patient with repeated tests for BNP from venous blood. These constraints prevent the monitoring of the BNP levels at home or at the office of the general practitioner and require that patients are directed to centers for laboratory investigations. To overcome these limitations, some systems suitable for allowing rapid and non-invasive measurement of multiple biological parameters, including the BNP [3], have been developed. Ideally, such a so-called “point-of-care” (POC) should measure the BNP from capillary blood, similarly to blood sugar measurement from the fingertip in diabetic patients. These POCs have many advantages, e.g., greater convenience and speed of detection of the results than in the reference methods. A system for detecting analytes at the bedside (POC) would enable easy and quick BNP monitoring even in a remote way, and would be useful for routine checks at the offices of the primary care physician or of the community cardiologist. In addition, POC may also be used in hospitals for the rapid monitoring of BNP-guided treatment of heart failure during an outpatient visit in the Department of Cardiology and for a rapid triage of patients with acute dyspnea in the Emergency Department. However, although promising, the preparation and evaluation of a POC as a diagnostic routine tool requires that this kind of method is conveniently assessed to test adequately its reliability in comparison with the reference methods, i.e. those used in laboratories for blood analyses. Until a short time ago, the two POCs available for the measurement of BNP were the AlereTM Triage [4] and the Abbott i-STAT [5, 6], the latter using venous or arterial whole blood, while the former uses plasma treated with ethylenediaminetetraacetic acid (EDTA) as a chelating agent (Table 1) [6-9]. In contrast, the AlereTM Heart Check BNP test is the first POC designed as a type of immunological assay for the measurement of BNP from whole untreated capillary blood. Two preview studies demonstrated the safety and feasibility of this test at home for patients [10] and healthcare providers [8]. However, no peer-reviewed studies have compared the AlereTM Heart Check BNP test with the reference methods used in the medical laboratory, except for the data provided by the AlereTM Heart Check BNP test compared with the Beckman Coulter Access II BNP assay [9]. Therefore, the aim of this study was to evaluate the clinical performance of this new POC to measure BNP by comparing it with our consolidated reference method (Abbott Architect System), as an analytical prerequisite for a large deployment of this system in the hospital.
 Click to view | Table 1. Comparison of the Analytical Performance of POC* and Lab-Based** BNP Assays [6, 8, 9] |
| Methods | ▴Top |
Patients
We enrolled 111 patients with stable CHF who were followed at the two cardiac rehabilitation units that participated in our search (E.d’A. and S.M.d.P.) from February 2013 to January 2015. For enrollment, only patients with CHF in New York Heart Association (NYHA) classes I-III were considered, irrespective of the origin (coronary artery disease, cardiomyopathy, valvular disease, etc.) of their cardiac insufficiency. Patients with a clinical picture of instability (i.e., patients with acute decompensated heart failure) were excluded. Both CHF patients with reduced left ventricular ejection fraction (LVEF) and those with preserved LVEF were included in the study. Heart failure was defined as the inability of the heart to pump sufficiently to maintain blood flow to meet the body’s needs. For clinical categorization’s purposes, we used the NYHA classes, according to the usual significance attributed to each class, as reported in Table 2.
 Click to view | Table 2. NYHA Classification |
BNP measurements
These patients were subjected to simultaneous capillary (AlereTM Heart Check System) and plasma (Abbott Architect System) BNP measurement. Plasma was obtained from EDTA blood samples centrifuged at 3,500 g for 15 min at 4 °C. Plasma BNP was subsequently measured on an Abbott Architect i2000. The AlereTM Heart Check BNP test used capillary whole blood to measure BNP. This method was a one-step immunoassay that uses biotinylated anti-BNP monoclonal antibody and streptavidin-coated magnetic solid-phase particles that were used to attract the immune binding of latex particles coupled with horseradish peroxidase and a monoclonal antibody fragment. This generated an electrochemical detection signal proportional to the level of BNP in the patient sample. The limits of detection were 10 - 4,955 pg/mL. The AlereTM Heart Check was ready to use on a static or mobile device; capillary blood obtained by pricking was deposited on a single-use test strip, and could be performed by patients themselves. There was no pre-analytical requisite for this method. Results were provided within 15 min. The Abbott Architect System was the lab-based system used in our hospitals for the routine measurement of plasma BNP; this method was valid and considered as a reference method. It was based on a two-step sandwich immunoassay using monoclonal antibodies specific for human BNP; detection was done by chemiluminescence. The limits of detection were 10 - 5,000 pg/mL. Unlike the AlereTM Heart Check, the Abbott Architect System required plasma to be obtained from EDTA whole blood by centrifugation at 3,500 g for 15 min at 4 °C, within 4 h after collection. Results were provided within 15 min.
Analytical performance: precision
Repeatability was assessed by performing six repeated measures of the low and high BNP controls on the same day by the same operator using strips and controls from the same batch. Reproducibility was assessed by performing six measures of the low and high BNP controls per day, over three consecutive days using three different strip batches.
Statistical analysis
All statistical analyses were performed using the Stata version 10 statistical software (StataCorp LP, College Station, TX, USA). Values were expressed as median (interquartile range), mean ± SD or number (%) as appropriate. The difference between capillary and plasma BNP was assessed by Mann-Whitney test (independent samples). Linear regression analysis was made for evaluating the relationship between the BNP values, respectively measured by means of the AlereTM Heart Check System and Abbott Architect System, the latter being used as reference method. Thus, the regression coefficient (slope) and the correlation coefficient concerning the two methods of BNP measurement were calculated. Bland-Altman plot was built for highlighting the difference between the two techniques for the calculated BNP value, by plotting this difference against the averages of the two techniques. Receiver operator characteristic curves were generated for evaluating the diagnostic accuracy of the two tests (AlereTM Heart Check and Abbott Architect). A P-value < 0.05 was considered significant.
| Results | ▴Top |
Clinical performance: characteristics of the study population
From February 2013 to January 2015, we enrolled 111 patients with stable CHF, mostly men (65%), whose characteristics are presented in Table 3. Fifty-four patients (48.6%) had a reduced ejection fraction (≤ 50%) mainly due to ischemic cardiac disease, and 49 (44%) had symptomatic heart failure (NYHA functional classes II-III). The patients underwent two types of blood sampling, one venipuncture and one fingertip capillary blood sample.
 Click to view | Table 3. Characteristics of the Study Population (n = 111) |
Comparison between AlereTM Heart Check BNP test and the reference method
Overall, the BNP values obtained ranged between 95 and 2,178 pg/mL using the AlereTM Heart Check, and between 89 and 1,435 pg/mL using the Architect System. There was a strong positive correlation between the two measurements (r = 0.947, P < 0.0001), although the values diverged more when BNP was higher than 1,500 pg/mL (Fig. 1). Bland-Altman analysis confirmed this difference (bias of 46.9 pg/mL), some values above 1,500 pg/mL being outside the 95% limit of agreement (Fig. 2).
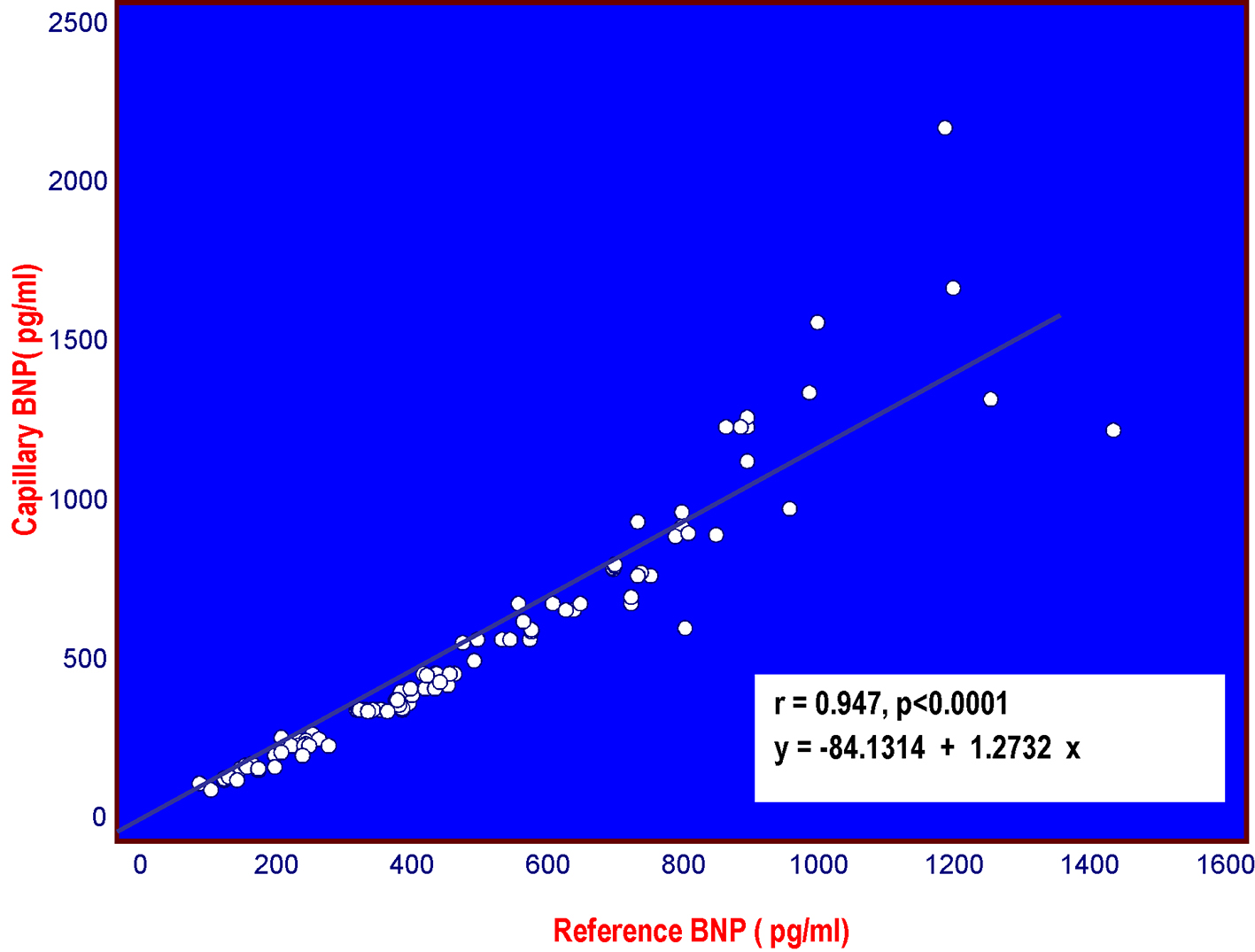 Click for large image | Figure 1. Distribution of capillary BNP measured on the Alere Heart Check system according to plasma BNP measured on the Abbott Architect System among the 111 patients with CHF, examined in the study. |
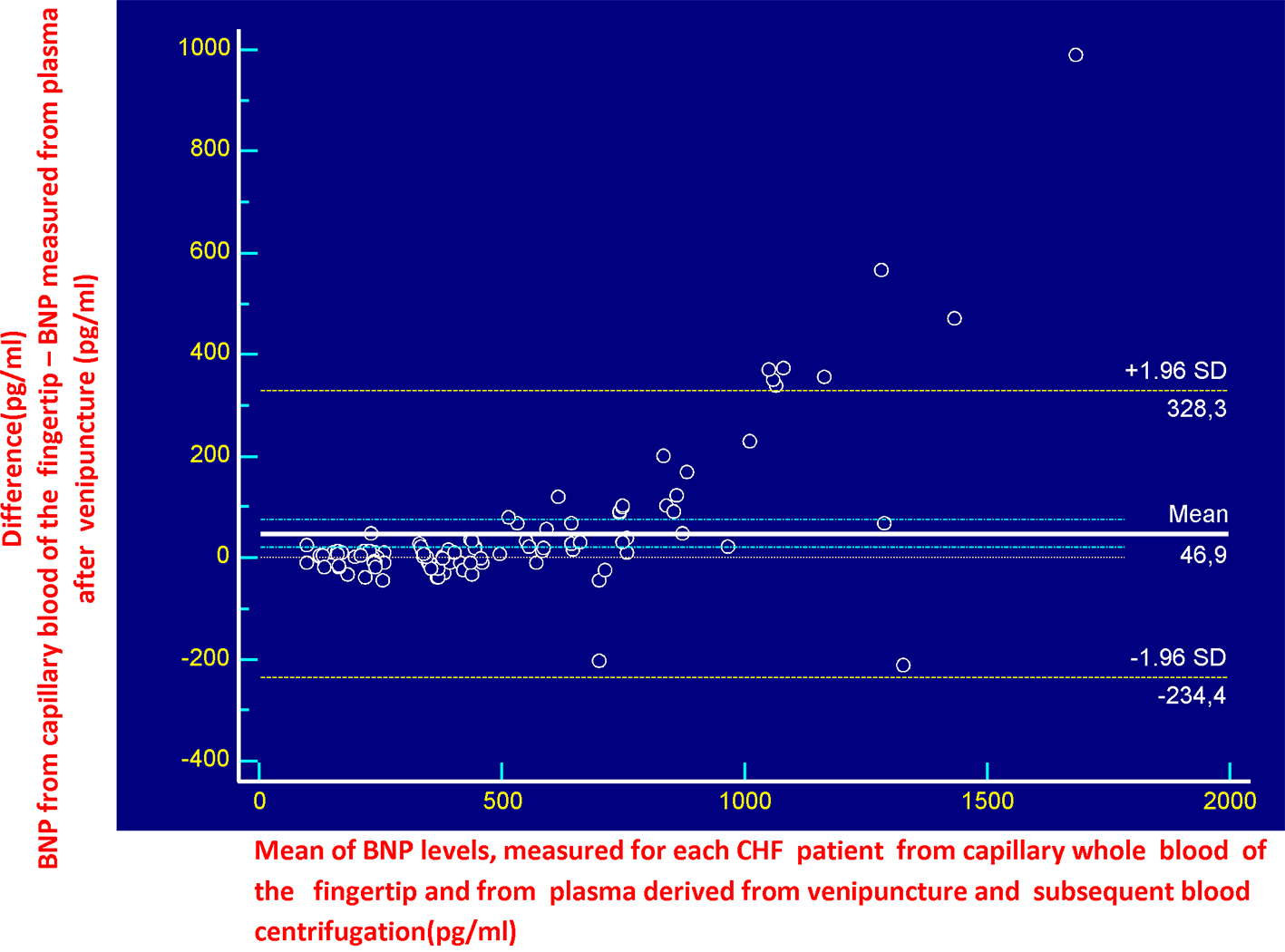 Click for large image | Figure 2. Bland-Altman comparison of capillary and plasma BNP. |
We compared both methods for their diagnostic accuracy in distinguishing the asymptomatic CHF patients, i.e. belonging to NYHA functional class I, from those with symptoms, i.e. belonging to NYHA functional classes II-III. For this purpose, we generated specific receiver operating characteristic curves. The methods (AlereTM Heart Check vs. Abbott Architect) showed similar diagnostic performances (Figs. 3 and 4) with values of area under the curve (AUC) of 0.983 and 0.984, respectively, and equivalent sensitivities, specificity, and positive and negative likelihood ratios.
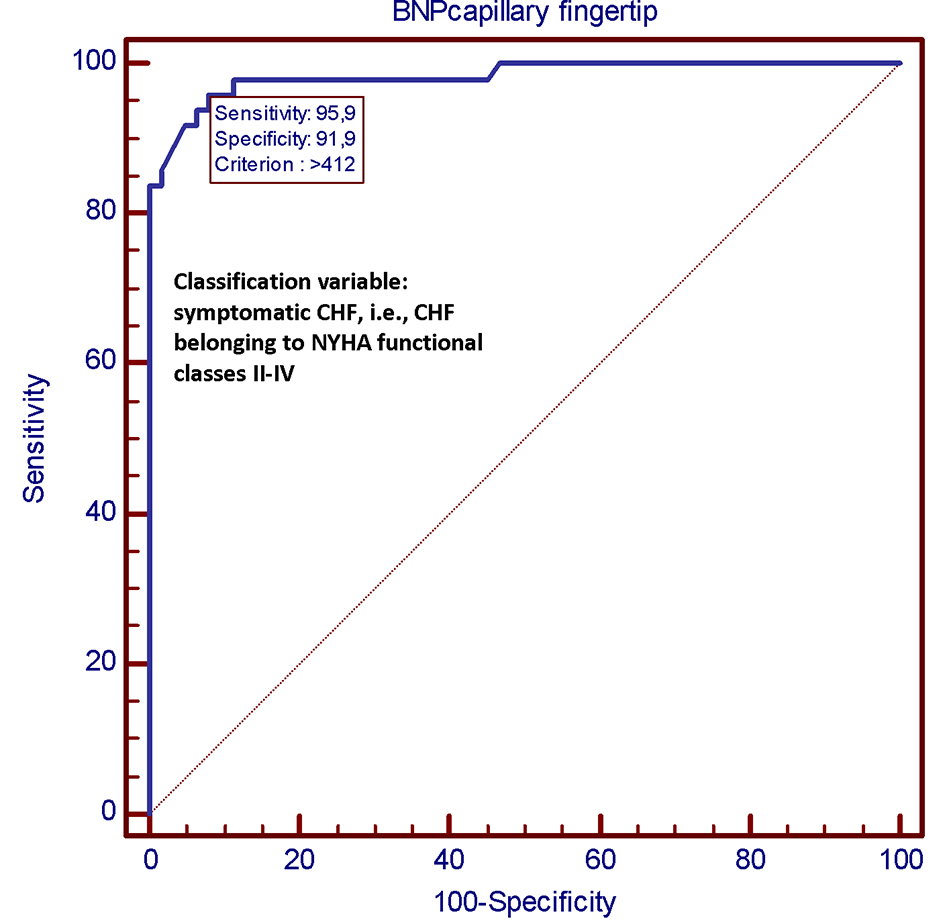 Click for large image | Figure 3. In this ROC plot, there is the representation of the very good diagnostic performance (AUC = 0.983) of the Alere Check System as a tool for predicting a clinical picture of CHF belonging to NYHA classes II-IV. By adopting this method, the best diagnostic accuracy for identifying a condition of NYHA class II or higher has been attributed to the BNP threshold value of 412 pg/mL. This means that this value, when derived from a measurement made by the Alere Check System on capillary blood, is associated to the presence of heart failure symptoms with a sensitivity of 95.9% and a specificity of 91.9% (note on top of the graph). CHF: chronic heart failure; NYHA: New York Heart Association; ROC: receiver operating characteristic; AUC: area under the curve; BNP: B-type natriuretic peptide; pg: picogram. |
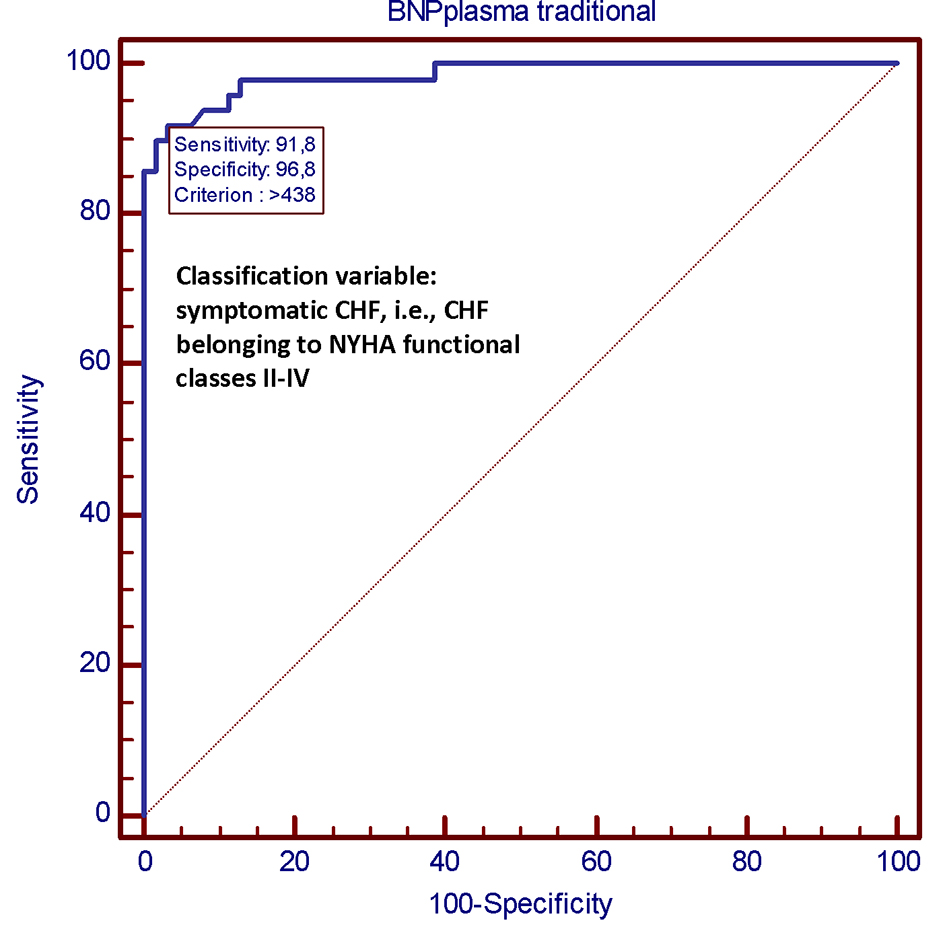 Click for large image | Figure 4. In this ROC plot, there is the representation of the very good diagnostic performance (AUC = 0.984) of the Abbott Architect System as a tool for predicting a clinical picture of CHF belonging to NYHA classes II-IV. The criterion value of 438 pg/mL has been identified as the BNP value that exhibits the best diagnostic accuracy for predicting the presence of symptoms of heart failure. This means that this value, when the BNP measurement is done from plasma using the Abbott Architect System, is associated with heart failure symptoms with the best combination of sensitivity and specificity (91.8% and 96.8%, respectively). CHF: chronic heart failure; NYHA: New York Heart Association; ROC: receiver operating characteristic; AUC: area under the curve; BNP: B-type natriuretic peptide; pg: picogram. |
We subsequently compared the results from capillary and plasma BNP measurements, according to the NYHA functional classification of dyspnea (Figs. 5-7). Below there are the pertinent findings, that we have found for each NYHA class by measuring the BNP levels in capillary whole blood (Alere Heart Check System) and EDTA plasma from venous sample (Abbott Architect System) respectively, expressed as median plus interquartile range. There was no statistical difference between capillary and plasma BNP according to the NYHA class of patients (NYHA I: 245 pg/mL (168.5 - 348.25) vs. 251 pg/mL (194 - 381); NYHA II: 610.5 pg/mL (500 - 765) vs. 578.5 pg/mL (495 - 699); NYHA III: 1,223 pg/mL (923.75 - 1,309.75) vs. 897 pg/mL (802.5 - 1,087.5)).
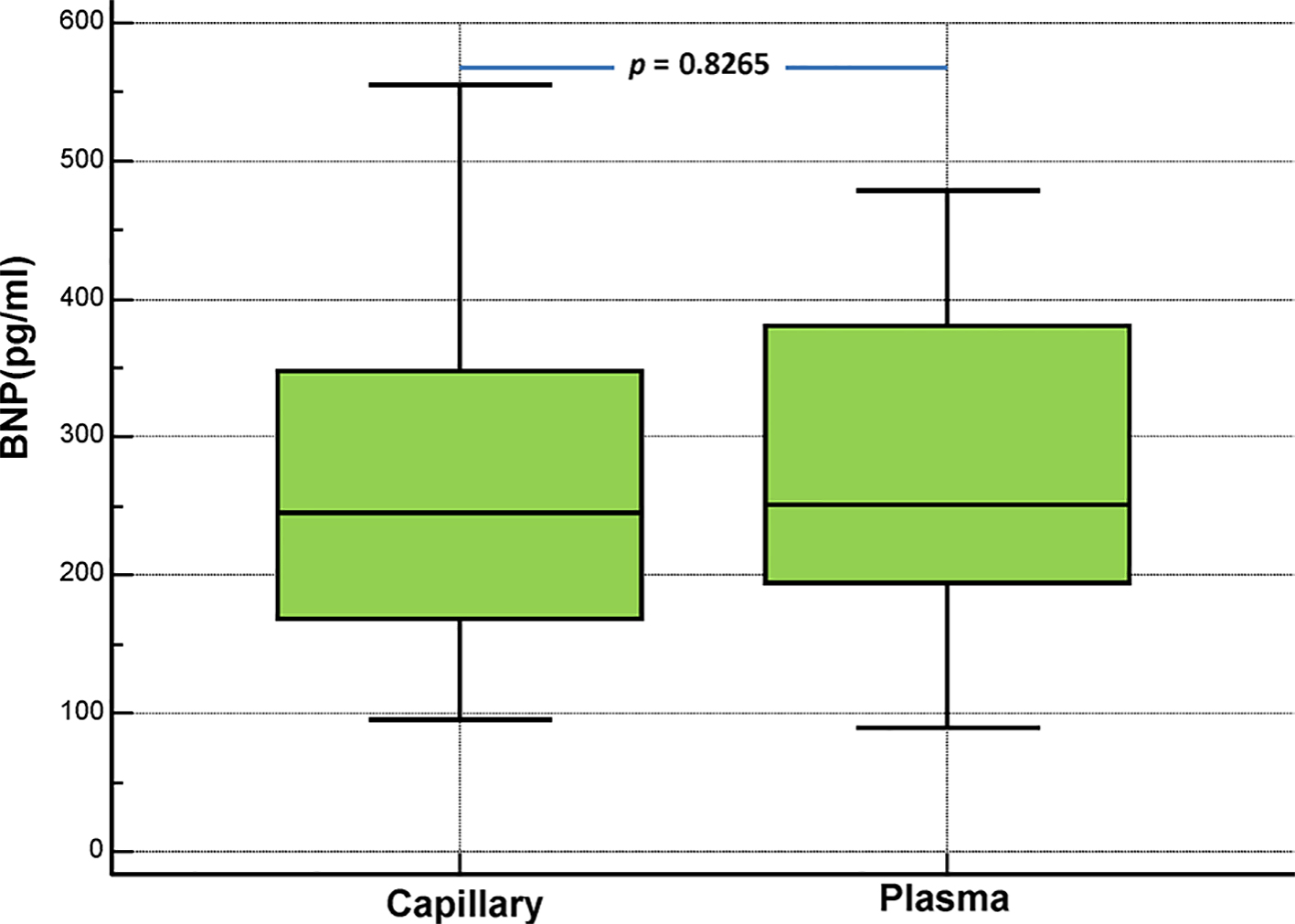 Click for large image | Figure 5. Comparison of capillary (Alere Heart Check System) and plasma (Abbott Architect System) BNP levels in patients with chronic heart failure NYHA class I (n = 62). |
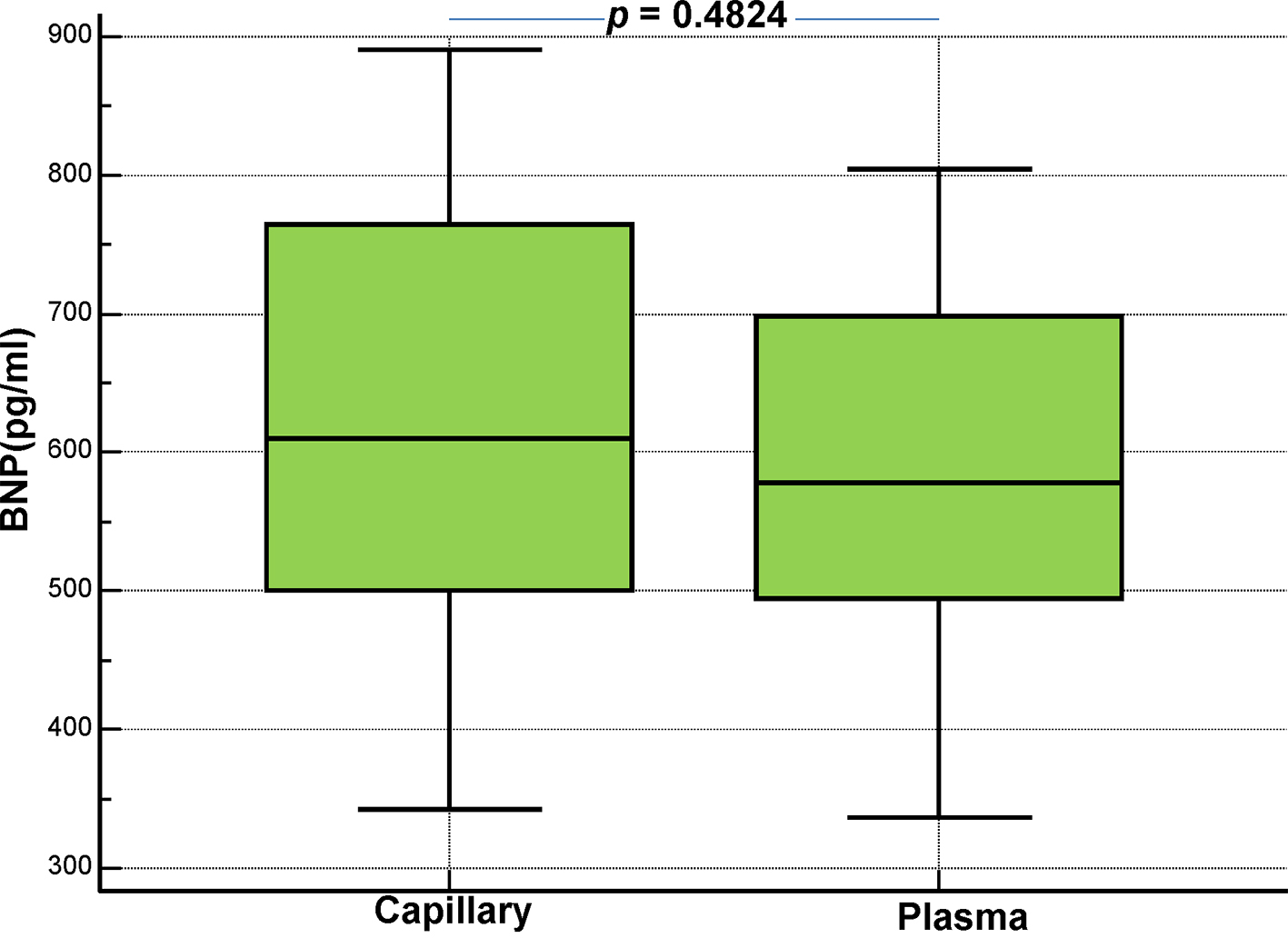 Click for large image | Figure 6. Comparison of capillary (Alere Heart Check System) and plasma (Abbott Architect System) BNP levels in chronic heart failure patients with NYHA class II (no = 30). |
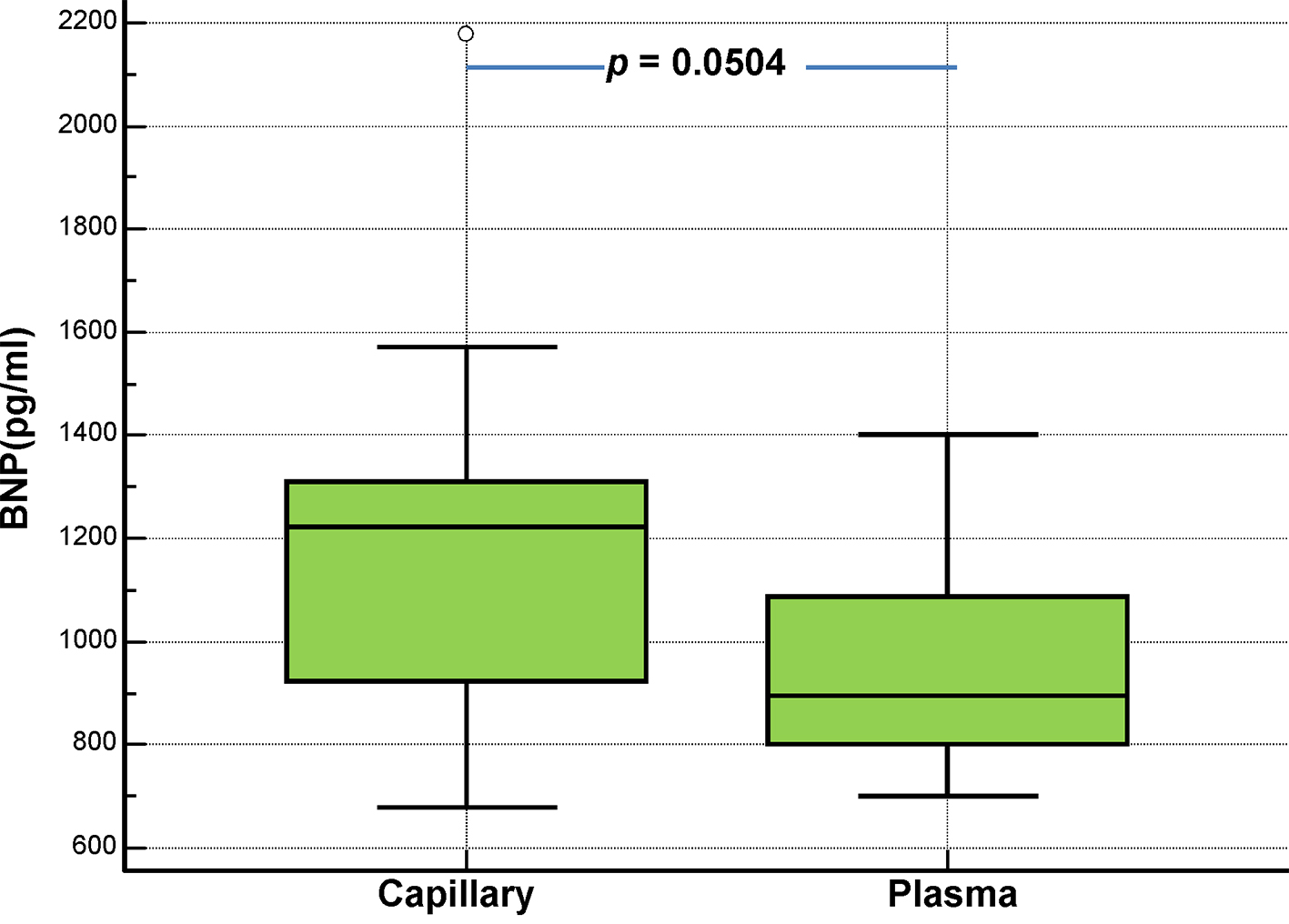 Click for large image | Figure 7. Comparison of capillary (Alere Heart Check System) and plasma (Abbott Architect System) BNP levels in chronic heart failure patients with NYHA class III (no = 19). |
| Discussion | ▴Top |
In this study, we evaluated the clinical performance of a new method for the hematochemical diagnosis of cardiac decompensation at the patient’s bedside, the AlereTM Heart Check system, by comparing it with our standard laboratory method (Abbott Architect), which measures the plasma BNP. Overall, the AlereTM Heart Check system showed good performance as evidenced by the good correlation with the results of the Abbott Architect found in the entire investigated cohort. Due to technical limitations, the POC testing methods are usually less accurate than the automata-based methods for quantifying biomarkers. The AlereTM Heart Check system is no exception, and its accuracy falls within the range observed for other POCs measuring BNP at low concentrations of BNP [5] (Table 1). For higher concentrations of BNP, the AlereTM Heart Check system exhibited a relatively high coefficient of variation (18%) compared to other POCs, which is in line with the correlation rather low between the values of capillary (AlereTM Heart Check) and plasma (Abbott Architect) BNP at higher concentrations (> 1,500 pg/mL), as found in NYHA class III (see also the box-and-whisker plots displayed in Figures 5-7). A similar variation, related to the concentration, was also observed in lab-based essays such as the i-STAT Abbott (Fig. 1). These results suggest that the AlereTM Heart Check system is an optimal tool for accurate identification of BNP levels, when they are located within its low-to-mid range. Overall, these data strongly suggest that the AlereTM Heart Check system is reliable for measuring BNP in patients with CHF, particularly those within NYHA classes I-III, although important changes to the BNP should be confirmed by lab-based tests. From a practical standpoint, this system is the only test for BNP from capillary blood; the Abbott i-STAT and the AlereTM Triage systems used in emergency departments measure BNP using either venipuncture EDTA whole blood (Abbott i-STAT) or EDTA plasma (AlereTM Triage). Therefore, the AlereTM Heart Check is the only non-invasive system that uses capillary blood from the fingertip and could easily be implemented as a rapid method for evaluating BNP by the general practitioner, the community cardiologist or for home monitoring, as evidenced by the recent HABIT trial [10]. The differences observed between the two methods (AlereTM Heart Check and Abbott Architect) are quite small, which suggests that both could be used, in alternation, to follow the efficacy of treatment in patients with CHF. Moreover, some ergonomic improvements could be made to facilitate the deposition of the blood droplet on the strip, which would result in fewer errors. In addition, the machine lacks traceability of the results since patients’ ID cannot be entered into the machine. Importantly, the data we obtained showed the AlereTM Heart Check BNP assay performed (AUC = 0.983) as well as the Abbott Architect assay (AUC = 0.984) in distinguishing patients with asymptomatic (NYHA I) and symptomatic (NYHA II-III) CHF. These results suggest that the AlereTM Heart Check BNP assay could be used in an emergency to triage dyspneic patients. However, our data suggest that more studies are needed to validate this method in emergency conditions. Indeed, capillary (AlereTM Heart Check) BNP was biased by 46.9 pg/mL (Bland-Altman plot in Fig. 2) compared to plasma (Abbott Architect) BNP. Currently, the algorithm available for the diagnosis of acute decompensated heart failure (ADHF) in international guidelines refers to a BNP cutoff of 100 pg/mL, i.e. ADHF can be excluded in acutely dyspneic patients whose BNP levels fall below this threshold value [1, 2]. Given the bias observed in our Bland-Altman comparison, the use of the AlereTM Heart Check system in emergency conditions will most likely overestimate patients who would fall into the gray zone of the BNP (100 - 400 pg/mL), among acutely dyspneic patients, unless a new threshold is determined for this system.
Our study has many limitations. First, it was a study based on only two participating centers, with a small population younger than the current population with CHF and a few women. However, the range of BNP measured was sufficiently broad to test the limits of the measurements. Compared to other POCs available for blood glucose, INR or plasma BNP, we postulate a higher cost than laboratory-based methods. However, cost-effectiveness should be evaluated in a larger-scale study, according to the aimed application, e.g. remote vs. in-hospital monitoring.
Conclusions
The AlereTM Heart Check BNP test is a good POC for the management of heart failure despite a relatively poor precision in higher BNP values. There was good agreement between the AlereTM Heart Check system and the lab-based test (Abbott Architect System). Thus, further studies are required to evaluate the real cost and real application of this device in emergency departments or by patients at home.
Financial Disclosures
There are no financial disclosures to be reported for the present article and the research work that is described in it.
| References | ▴Top |
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787-1847.
doi pubmed - Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr., Drazner MH, Fonarow GC, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-239.
doi pubmed - Cheng V, Kazanagra R, Garcia A, Lenert L, Krishnaswamy P, Gardetto N, Clopton P, et al. A rapid bedside test for B-type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol. 2001;37(2):386-391.
doi - Vogeser M, Jacob K. B-type natriuretic peptide (BNP) - validation of an immediate response assay. Clin Lab. 2001;47(1-2):29-33.
pubmed - Ro R, Thode HC, Jr., Taylor M, Gulla J, Tetrault E, Singer AJ. Comparison of the diagnostic characteristics of two B-type natriuretic peptide point-of-care devices. J Emerg Med. 2011;41(6):661-667.
doi pubmed - Shah K, Terracciano GJ, Jiang K, Maisel AS, Fitzgerald RL. Comparability of Results between Point-of-Care and Automated Instruments to Measure B-type Natriuretic Peptide. West J Emerg Med. 2010;11(1):44-48.
pubmed - Heartcheck BNP test strip package insert 0017 spec-0363 rev. 1 2010/09, Alere Technologies Ltd, Stirling, Scotland.
- Lang NN, Wong CM, Dalzell JR, Jansz S, Leslie SJ, Gardner RS. The ease of use and reproducibility of the Alere Heart Check System: a comparison of patient and healthcare professional measurement of BNP. Biomark Med. 2014;8(6):791-796.
doi pubmed - Triage® BNP test product insert, 2011, Alere Technologies Ltd., Stirling, Scotland.
- Maisel A, Barnard D, Jaski B, Frivold G, Marais J, Azer M, Miyamoto MI, et al. Primary results of the HABIT Trial (heart failure assessment with BNP in the home). J Am Coll Cardiol. 2013;61(16):1726-1735.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


