| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website http://www.cardiologyres.org |
Original Article
Volume 8, Number 6, December 2017, pages 286-292
Transcatheter Closure Versus Repeat Surgery for the Treatment of Postoperative Left-to-Right Shunts: A Single Center 15-Year Experience
Xinghua Gua, Qiuwang Zhangb, Hourong Suna, Jianchun Feia, Xiquan Zhanga, c, Michael J. Kutrykb
aDepartment of Cardiovascular Surgery, Qilu Hospital of Shandong University, 107 Wenhua West Road, Jinan 250012, China
bDivision of Cardiology, Keenan Research Center for Biomedical Science at the Li Ka Shing Knowledge Institute, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada
cCorresponding Author: Xiquan Zhang, Department of Cardiovascular Surgery, Qilu Hospital of Shandong University, 107 Wenhua West Road, Jinan 250012, China
Manuscript submitted November 5, 2017, accepted December 5, 2017
Short title: Treatment of Postoperative Left-to-Right Shunts
doi: https://doi.org/10.14740/cr629e
| Abstract | ▴Top |
Background: Repeat surgery and the percutaneous approach (transcatheter closure (TCC)) have been used for the management of postoperative left-to-right shunts. In this study, we described our 15 years of experience in treating postoperative left-to-right shunts with these two approaches.
Methods: From February 2002 to February 2017, 50 patients with residual left-to-right shunts, following cardiac surgery, were treated using TCC or repeat surgery. Clinical examination, standard 12-lead electrocardiography, chest X-ray, and a transthoracic echocardiogram were performed before hospital discharge and at all follow-ups.
Results: The closure rate was 100% in both groups and there was no procedure-related mortality. Patients with TCC had few complications. The procedure time and duration of hospital stay for TCC patients were 58.9 ± 27.7 min and 6.1 ± 0.8 days, respectively. Eleven out of 19 patients receiving reoperation suffered serious complications after surgery, e.g., bleeding and nosocomial infections. The operation time and duration of hospital stay for reoperation patients were 256.7 ± 60.5 min and 17.0 ± 4.0 days, respectively. No other serious complications were seen at all follow-up visits for both groups.
Conclusions: In conclusions, TCC is safe and effective for the management of postoperative left-to-right shunts, and is associated with few complications, which can be the favored closure strategy over repeat surgery for the management of postoperative left-to-right shunts.
Keywords: Postoperative left-to-right shunts; Transcatheter closure; Repeat surgery
| Introduction | ▴Top |
Although significantly decreased due to the improvement of operative techniques, left-to-right shunts, e.g., residual ventricular septal defect (VSD), residual patent ductus arteriosus (PDA) and new-onset left-to-right shunts still occur in patients after cardiac surgery [1-5]. While the majority of postoperative left-to-right shunts do not need to be treated [1], some of these shunts require re-intervention as they result in left ventricular volume overload, elevated pulmonary vascular resistance and predispose to infective endocarditis [2-9]. It is generally accepted that a Qp/Qs ≥ 1.5, or the presence of clinical symptoms, are indications for re-intervention [10, 11]. In this study, we present our 15 years experience in the management of postoperative left-to-right shunts with transcatheter closure (TCC) and repeat surgery.
| Patients and Methods | ▴Top |
From February 2002 to February 2017, a total of 50 patients with residual left-to-right shunts following cardiac surgery were treated with either TCC or repeat surgery at our center. Written informed consent was obtained from all patients prior to re-intervention. Collection and use of clinical data were approved by the Research Ethics Committee, Qilu Hospital of Shandong University.
Transcatheter procedure
The procedure was performed under local or general anesthesia (general anesthesia was performed for patients under the age of 12 years) with fluoroscopic and transthoracic echocardiogram (TTE) guidance. Patients were given 100 IU/kg heparin intravenously after femoral venous and arterial access.
For closure of residual VSDs, standard right heart catheterization, angiography of left ventricle and ascending aorta were performed in all cases according to the techniques described previously [12]. Perimembranous VSD (pmVSD) occluders (Lifetech Ltd, Shenzhen, China) approximately 2 - 3 mm greater than the shunt size, were selected. The arterio-venous circuit was created, and the device was deployed under fluoroscopic control. Successful closures were made when the device was implanted in the appropriate position without significant complications.
For closure of residual PDAs, PDA occluders (Lifetech Ltd, Shenzhen, China) sized approximately 2 - 3 mm greater than the narrowest portion of PDA were used. As described previously [13], the delivery sheath was positioned anterogradely from the main pulmonary artery through PDA into the descending aorta. The device was then deployed under fluoroscopic control, and released once the final position was assessed. When it was difficult to direct the multipurpose catheter through the PDA in the anterograde approach, the retrograde wire-assisted technique was used through the establishment of an arteriovenous wire loop as described elsewhere [14].
In our series, two patients had new-onset ruptured sinus of Valsalva aneurysms (RSVAs) with one originating from the non-coronary artery sinus and ruptured into the right atrium (RSVA-RA), and the other originating from the right coronary sinus and into the right ventricular outflow tract (RSVA-RVOT). For closure of new-onset RSVA, routine right and left cardiac catheterization was performed to obtain hemodynamic data. Aortic root angiography was performed in two views; the left anterior oblique with cranial tilt (LAO 60, 20 - 30 cranial) and the right anterior oblique (RAO 30), to delineate the RSVA, the origin of the RSVA, and its fistulous connections to the cardiac chambers. The stable arterial-venous wire loop was then established via the RSVA. A pmVSD occluder for RSVA-RVOT and a PDA occluder (Lifetech Ltd, Shenzhen, China) for RSVA-RA were used, respectively. Under the guidance of fluoroscopy and TTE, the device was deployed at the opening of the RSVA. Aortography and TTE were repeated to confirm that the RSVA was closed completely, and that there was no significant aortic or tricuspid regurgitation. Coronary angiography was performed to ensure that there was no encroachment on the coronary arteries, and then the device was released [5].
Surgical procedure
Median re-sternotomy was performed in all patients. Cardiopulmonary bypass with moderate systemic hypothermia was established either through the femoral vessels or the subclavian artery and femoral vein. After the ascending aorta was cross-clamped, the cold blood cardioplegia was infused into the root of the aorta until diastolic arrest was achieved. Residual VSD was exposed through the right atrium or the right ventricular outflow tract, and repaired with either direct sutures or patch of Dacron (interrupted pledgeted sutures or a continuous suture).
Follow-up protocol
All patients underwent clinical examination and telemetry monitoring for 24 h after shunt closure. Aspirin was prescribed 3 - 5 mg/kg/day for those with TCC for 6 months post procedure. Clinical examination, standard 12-lead electrocardiography (ECG), chest X-ray, and TTE were performed before hospital discharge, and at 1, 3, 6, and 12 months post procedure and yearly thereafter.
Statistical analysis
The continuous data between the two groups were compared and analyzed with the Student’s t-test. The categorical data were analyzed by the Chi-square test. P < 0.05 was considered significantly different.
| Results | ▴Top |
Patients’ follow-up ranged from 0.5 to 15.5 years. The demographics and clinical characteristics of the patients, undergoing TCC or reoperation, are listed in Tables 1 and 2, respectively. There was no significant difference in age, gender or shunt size between the two groups. For those with TCC, 25 had a residual VSD, four patients had a residual PDA and two patients had new-onset RSVA (one with RSVA-RVOT and the other with RSVA-RA). The procedures of TCC of residual VSD, residual PDA and RSVA-RVOT are depicted in Figures 1, 2 and 3, respectively. All 19 patients in the repeat surgery group had residual VSD. There were no procedure-related deaths in either group. In the TCC group, one patient had incomplete right bundle branch block, and two patients had trivial post-procedural intraprosthetic residual shunts that disappeared by 3 months follow-up. There was no atrioventricular block (AVB), hemolysis, new-onset residual shunt, device embolization, device dislocation, infective endocarditis, or new-onset aortic/tricuspid regurgitation seen in the TCC patients. In the surgical group, complications following surgery included bleeding requiring peri/post-procedural blood transfusion (11/19, 57.9%), nosocomial infection (3/19, 15.8%), wound dehiscence (2/19, 10.5%) and renal insufficiency (1/19, 5.3%) (Table 2).
 Click to view | Table 1. Demographics and Clinical Characteristics of Patients Undergoing Transcatheter Closure |
 Click to view | Table 2. Demographics and Clinical Characteristics of Patients Treated With Reoperation |
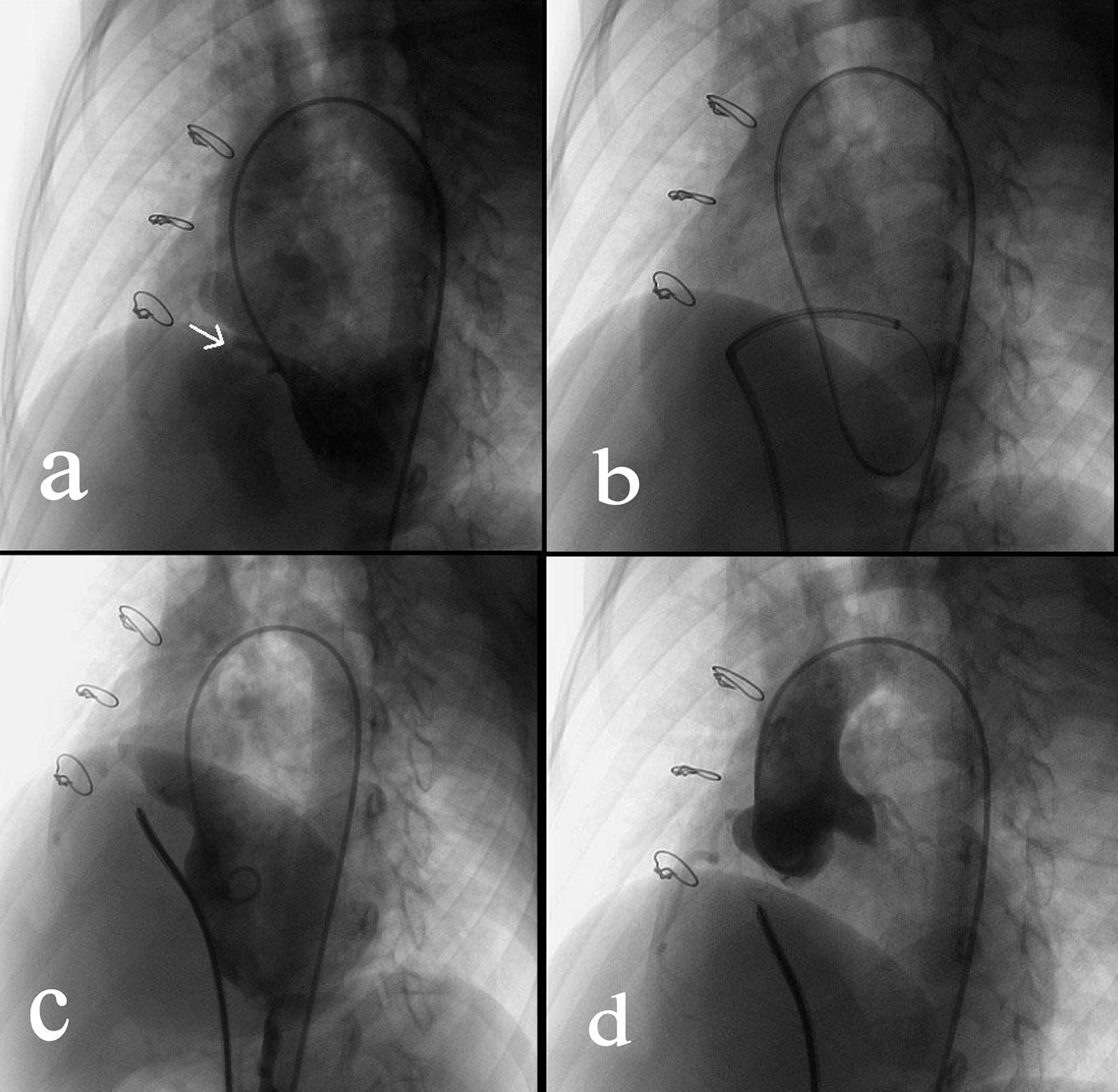 Click for large image | Figure 1. Transcatheter closure of a residual VSD. (a) Angiography of left ventricle via a 4F pigtail catheter inserted through the right femoral artery showed the residual VSD (indicated by the arrow). (b) Via the right femoral vein, a femoral vein-inferior vena cava-right ventricle-residual VSD-left ventricle-aorta-right femoral artery loop was established using a 260 cm loach guide wire. Afterwards, a 6-F delivery catheter was advanced along the loop to the left ventricle. (c) The loop wire was withdrawn. Subsequently, a VSD occluder was delivered to left ventricle and then opened in following sequence: left disc, waist and right disc. (d) After repeated angiography showed that the shunt disappeared, and then the occluder was released. |
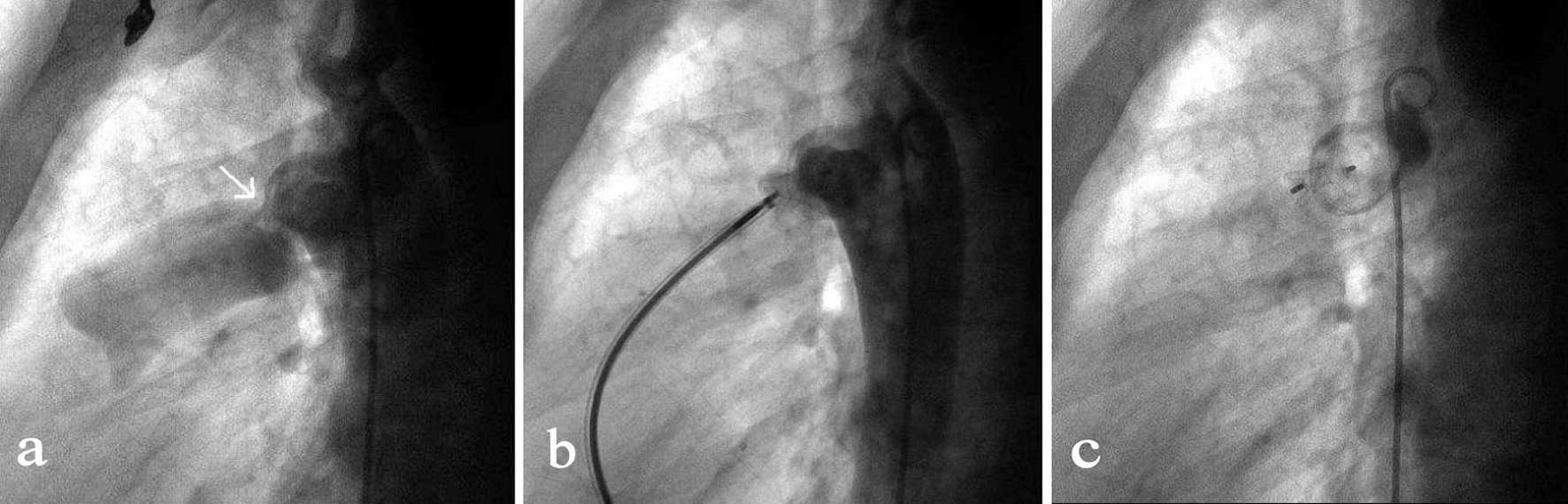 Click for large image | Figure 2. Transcatheter closure of a residual PDA. (a) Angiography of the aortic arch via a 5-F pigtail catheter that was inserted through the right femoral artery showed the residual PDA shunt (indicated by the arrow). (b) By the access of right femoral vein, a MPA2 catheter was advanced via right ventricle, pulmonary artery, PDA to the descending aorta followed by the insert of a 260 mm wire. After the MPA2 catheter was withdrawn, an 8-F delivery sheath was advanced along the wire and the duct occluder was delivered, positioned and opened in such a sequence that the aortic end of the occluder was first opened and then the pulmonary artery end. (c) Repeated angiography showed the occluder was properly placed and the shunt disappeared, and then the duct occluder was released. |
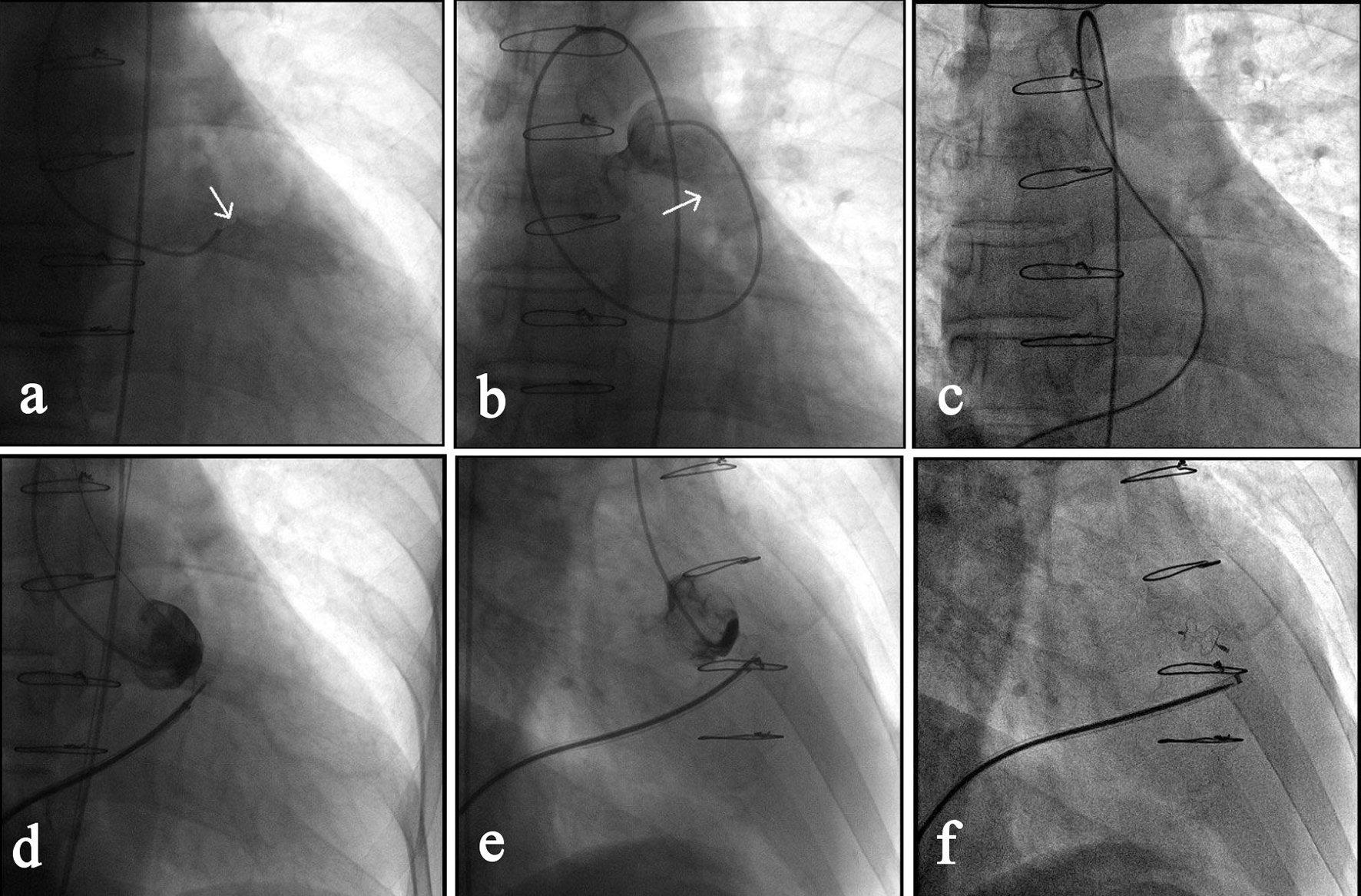 Click for large image | Figure 3. Transcatheter closure of a RSVA. (a) Angiography of right coronary sinus via a MPA1 catheter that was inserted into the right femoral artery showed the shunt from right coronary sinus to the right ventricular outflow tract (as indicated by the arrow). (b) Angiography after the MPA1 catheter was further advanced to the pulmonary artery confirmed the shunt (arrow). (c) Via the right femoral vein, a femoral vein-inferior vena cava-right ventricle-RSVA-aorta-right femoral artery loop was established using a 260 cm loach guide wire. (d) A 7-F delivery sheath was advanced along the loop to ascending aorta, and then a 7 mm VSD occluder was delivered passing the RSVA and opened in following sequence: left disc, waist and right disc. (e) The loop wire was withdrawn and angiography showed the shunt disappeared. (f) The occluder was released, delivery catheter withdrawn and repeated angiography showed the occluder was properly placed. |
The data from all patients is further summarized in Table 3. After a hybrid operating room was established and became operational at our center in 2010, 85.7% (30/35) of patients have received percutaneous intervention. Prior to opening the hybrid room, 93.3% of patients (14/15) underwent reoperation. The procedure time for TCC was 58.9 ± 27.7 min, which was significantly shorter than 256.7 ± 60.5 min of the reoperation group (P < 0.001). The length of hospital stay for patients with TCC was 6.1 ± 0.8 days, also significantly shorter compared with that of the reoperation group (P < 0.001).
 Click to view | Table 3. Summarized Data for Patients Undergoing TCC and Reoperation |
| Discussion | ▴Top |
During a period of 15 years, a total of 50 patients with postoperative left-to-right shunts were treated with TCC or repeat surgery at our center. Among all cases treated, the majority had a residual VSD (88%, 44/50). Surgery is regarded as the standard of care for most VSDs [1, 2], but reoperation requires cardiopulmonary bypass, and is associated with hemodynamic instability, shunt recurrence, morbidity, and mortality [2, 15]. In the surgical group, VSD closure was 100% successful, but, post-surgery complications were seen in over half of the patients. Additionally, procedure time and hospital stay for patients who had undergone surgical closure were markedly longer than those in the TCC group.
TCC has been reported to be safe and effective in the closure of both congenital and postoperative VSDs [6-9, 16-20]. Knauth et al summarized their 13-year experience in treating congenital and residual VSDs using STARFlex-type devices [6]. They implanted the device successfully in 77 out of a total of 78 patients with residual VSDs, but observed that adverse events, mainly caused by device malposition, were common. Walsh et al performed TCC for postoperative VSDs with the Amplatzer device in nine patients, and observed complete closure in six cases, and a small residual shunt in three cases [7]. No serious complications were seen during follow-up (1 - 4 years). Dua et al reported the early and mid-term (median follow-up: 2.7 years) results of TCC of residual VSDs using the Amplatzer VSD occluder in 22 patients. The success rate was 95.5% (21/22) with three minor adverse events being observed before discharge. There was no procedure-related mortality and no late events during follow-ups [8]. Zhang used the Amplatzer VSD occluder to manage residual VSD in 21 patients and attained a 100% occlusion rate with one having serious intravascular hemolysis that was recovered after 7 days of therapy [9]. No serious complications occurred during 1 year follow-up. These data, together with ours, suggest that the Amplatzer device is preferred to reduce complications [7-9, 19].
TCC has overwhelmingly replaced surgical treatment for every type of PDA in different age groups, except in neonates or small infants with large symptomatic PDAs [13, 21]. Coil implantation is the best option for treatment of small shunts (< 2 mm) [21-23], and for larger shunts the Amplatzer duct occluder appears to be superior [21, 24]. A residual PDA tends to be less distensible than a previously untreated one due to reactive fibrosis around the duct. This makes a TCC more difficult and a retrograde or snare technique is often used instead of a standard anterograde approach. In this study, the snare technique was used in two of the four patients with a residual PDA. TCC was successful in all patients with a residual PDA, without complications.
TCC is a relatively new treatment modality for isolated RSVA. Thus far, specific devices for RSVA closure are not available, and devices such as the Gianturco coil, the VSD occlude, and the Amplatzer and other duct occluders have been used. Of these different devices, duct occluders are the most commonly used and have been proven to be safe and effective [25-28]. Fang et al reported the long-term outcomes of TCC using a PDA occluder (Lifetech Ltd, Shenzhen, China) in 17 patients [27]. The procedural success rate was 94.1%, and all patients had complete occlusion at discharge. There were no late complications during a median follow-up of 42 months. Kerkar et al reported procedural success in 18 out of 20 patients treated with the Amplatzer duct occluder. Thirteen had complete closure, and five patients had mild residual shunts at discharge [28]. In our series, two patients suffered new-onset RSVA after VSD repair, with one being RSVA-RA and the other being RSVA-RVOT. The PDA occluder was used in the patient with RSVA-RA. Considering the possibility that the PDA device may cause iatrogenic RVOT obstruction, a VSD occluder was chosen and implanted successfully for the patient with RSVA-RVOT.
Conclusions
Our experience with TCC closure of postoperative left-to-right shunts adds to the accumulating evidence of high occlusion rates with few serious complications and supports the opinion that TCC is the favored closure strategy over repeat surgery in most instances. Careful preoperative planning and multi-modal cardiac imaging in a hybrid operating suite are critically important for safe and successful TCC of postoperative left-to-right shunts. A limitation of this study is that this is a single center experience.
Conflict of Interest
None.
Funding Support
This study was funded in part by the Independent Innovation Foundation of Shandong University (grant number 2012TS171) and the Research Fund for Outstanding Young Scientists of Shandong Province (grant number 2006BS03014), China.
| References | ▴Top |
- Bol-Raap G, Weerheim J, Kappetein AP, Witsenburg M, Bogers AJ. Follow-up after surgical closure of congenital ventricular septal defect. Eur J Cardiothorac Surg. 2003;24(4):511-515.
doi - Roos-Hesselink JW, Meijboom FJ, Spitaels SE, Van Domburg R, Van Rijen EH, Utens EM, Bogers AJ, et al. Outcome of patients after surgical closure of ventricular septal defect at young age: longitudinal follow-up of 22-34 years. Eur Heart J. 2004;25(12):1057-1062.
doi pubmed - Kusa J, Szkutnik M, Czerpak B, Bialkowski J. Percutaneous closure of previously surgical treated arterial ducts. EuroIntervention. 2008;3(5):584-587.
doi pubmed - Baspinar O, Kilinc M, Kervancioglu M, Irdem A. Transcatheter closure of a residual patent ductus arteriosus after surgical ligation in children. Korean Circ J. 2011;41(11):654-657.
doi pubmed - Schaeffler R, Sarikouch S, Peuster M. Transcatheter closure of a ruptured sinus of Valsalva aneurysm (RSVA) after aortic valve replacement using the Amplatzer muscular VSD Occluder. Clin Res Cardiol. 2007;96(12):904-906.
doi pubmed - Knauth AL, Lock JE, Perry SB, McElhinney DB, Gauvreau K, Landzberg MJ, Rome JJ, et al. Transcatheter device closure of congenital and postoperative residual ventricular septal defects. Circulation. 2004;110(5):501-507.
doi pubmed - Walsh MA, Coleman DM, Oslizlok P, Walsh KP. Percutaneous closure of postoperative ventricular septal defects with the Amplatzer device. Catheter Cardiovasc Interv. 2006;67(3):445-451; discussion 452.
doi pubmed - Dua JS, Carminati M, Lucente M, Piazza L, Chessa M, Negura D, Bussadori C, et al. Transcatheter closure of postsurgical residual ventricular septal defects: early and mid-term results. Catheter Cardiovasc Interv. 2010;75(2):246-255.
doi pubmed - Zhang B, Liang J, Zheng X, Jiang G, Yang Z, Zhang L, Zhang Y, et al. Transcatheter closure of postoperative residual ventricular septal defects using Amplatzer-type perimembranous VSD occluders. J Invasive Cardiol. 2013;25(8):402-405.
pubmed - Preminger TJ, Sanders SP, van der Velde ME, Castaneda AR, Lock JE. "Intramural" residual interventricular defects after repair of conotruncal malformations. Circulation. 1994;89(1):236-242.
doi pubmed - Ammash NM, Warnes CA. Ventricular septal defects in adults. Ann Intern Med. 2001;135(9):812-824.
doi - Rao PS. Perimembranous ventricular septal defect closure with the amplatzer device. J Invasive Cardiol. 2008;20(5):217-218.
pubmed - Moore JW, Levi DS, Moore SD, Schneider DJ, Berdjis F. Interventional treatment of patent ductus arteriosus in 2004. Catheter Cardiovasc Interv. 2005;64(1):91-101.
doi pubmed - Hsin HT, Lin LC, Hwang JJ, Ho SG, Tseng CD, Chiang FT. Retrograde wire-assisted percutaneous transcatheter closure of persistent ductus arteriosus with Amplatzer duct occluder in the elderly: A new application. Catheter Cardiovasc Interv. 2004;61(2):264-267.
doi pubmed - Said SM, Dearani JA. Strategies for high-risk reoperations in congenital heart disease. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2014;17(1):9-21.
doi pubmed - Carminati M, Butera G, Chessa M, Drago M, Negura D, Piazza L. Transcatheter closure of congenital ventricular septal defect with Amplatzer septal occluders. Am J Cardiol. 2005;96(12A):52L-58L.
doi pubmed - Chessa M, Butera G, Negura D, Bussadori C, Giamberti A, Fesslova V, Carminati M. Transcatheter closure of congenital ventricular septal defects in adult: mid-term results and complications. Int J Cardiol. 2009;133(1):70-73.
doi pubmed - Pedra CA, Pontes SC, Jr., Pedra SR, Salerno L, Sousa JB, Miaira MA, Guerra AL, et al. Percutaneous closure of postoperative and post-traumatic ventricular septal defects. J Invasive Cardiol. 2007;19(11):491-495.
pubmed - Michel-Behnke I, Le TP, Waldecker B, Akintuerk H, Valeske K, Schranz D. Percutaneous closure of congenital and acquired ventricular septal defects - considerations on selection of the occlusion device. J Interv Cardiol. 2005;18(2):89-99.
doi pubmed - Yang J, Yang L, Wan Y, Zuo J, Zhang J, Chen W, Li J, et al. Transcatheter device closure of perimembranous ventricular septal defects: mid-term outcomes. Eur Heart J. 2010;31(18):2238-2245.
doi pubmed - Baruteau AE, Hascoet S, Baruteau J, Boudjemline Y, Lambert V, Angel CY, Belli E, et al. Transcatheter closure of patent ductus arteriosus: past, present and future. Arch Cardiovasc Dis. 2014;107(2):122-132.
doi pubmed - Cambier PA, Kirby WC, Wortham DC, Moore JW. Percutaneous closure of the small (less than 2.5 mm) patent ductus arteriosus using coil embolization. Am J Cardiol. 1992;69(8):815-816.
doi - Takata H, Higaki T, Sugiyama H, Kitano M, Yamamoto E, Nakano T, Nagashima M, et al. Long-term outcome of coil occlusion in patients with patent ductus arteriosus. Circ J. 2011;75(2):407-412.
doi pubmed - Wang JK, Wu MH, Hwang JJ, Chiang FT, Lin MT, Lue HC. Transcatheter closure of moderate to large patent ductus arteriosus with the Amplatzer duct occluder. Catheter Cardiovasc Interv. 2007;69(4):572-578.
doi pubmed - Sen S, Chattopadhyay A, Ray M, Bandyopadhyay B. Transcatheter device closure of ruptured sinus of Valsalva: Immediate results and short term follow up. Ann Pediatr Cardiol. 2009;2(1):79-82.
doi pubmed - Guan L, Zhou D, Zhang F, Pan W, Dong L, Chen H, Ge J. Percutaneous device closure of ruptured sinus of valsalva aneurysm: a preliminary experience. J Invasive Cardiol. 2013;25(10):492-496.
pubmed - Fang ZF, Huang YY, Tang L, Hu XQ, Shen XQ, Tang JJ, Zhou SH. Long-term outcomes of transcatheter closure of ruptured sinus valsalva aneurysms using patent ductus arteriosus occluders. Circ J. 2014;78(9):2197-2202.
doi pubmed - Kerkar PG, Lanjewar CP, Mishra N, Nyayadhish P, Mammen I. Transcatheter closure of ruptured sinus of Valsalva aneurysm using the Amplatzer duct occluder: immediate results and mid-term follow-up. Eur Heart J. 2010;31(23):2881-2887.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


