| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website http://www.cardiologyres.org |
Original Article
Volume 10, Number 3, June 2019, pages 135-141
Prevalence and Clinical Implication of Wellens’ Sign in Patients With Non-ST-Segment Elevation Myocardial Infarction
Akihiro Kobayashia, c, Naoki Misumidaa, Shunsuke Aoia, Yumiko Kaneib
aDepartment of Internal Medicine, Mount Sinai Beth Israel, New York, NY, USA
bDepartment of Cardiology, Mount Sinai Beth Israel, New York, NY, USA
cCorresponding Author: Akihiro Kobayashi, Department of Internal Medicine, Mount Sinai Beth Israel, First Avenue at 16th Street, New York, NY 10003, USA
Manuscript submitted March 4, 2019, accepted March 26, 2019
Short title: Wellens’ Sign in Patients With NSTEMI
doi: https://doi.org/10.14740/cr856
| Abstract | ▴Top |
Background: Symmetrically inverted or biphasic T waves in anterior precordial leads, Wellens’ sign, have been shown to represent impending infarction of left anterior descending (LAD) territory among unstable angina patients in the studies published more than 3 decades ago, when non-ST-segment elevation myocardial infarction (NSTEMI) was not a recognized entity. The clinical implication of Wellens’ sign in the contemporary NSTEMI cohort has not been clarified.
Methods: We performed a retrospective analysis of all NSTEMI patients who underwent coronary angiography between January 2013 and June 2014. Wellens’ sign was defined as either symmetrically inverted T waves (≥ 0.10 mV) or biphasic T waves in both leads V2 and V3. Coronary angiograms were reviewed and culprit lesions were determined for each patient.
Results: A total of 274 patients were included in the final analysis, of whom 24 (8.8%) had Wellens’ sign. Among these 24 patients, 16 had a LAD culprit (eight proximal), two had a non-LAD culprit, and six had non-obstructive coronary artery disease. Patients with Wellens’ sign were more likely to have LAD culprit (66.7% vs. 19.6%, P < 0.001) and proximal LAD culprit (33.3% vs. 14.4%, P = 0.035) than those without it. Wellens’ sign had a sensitivity of 24.6% and a specificity of 96.2% to predict LAD culprit.
Conclusions: Our study revealed that: 1) Wellens’ sign was seen in 8.8% of the patients with NSTEMI; 2) Two-thirds of patients with Wellens’ sign had LAD culprit and one-third had proximal LAD culprit; and 3) Sensitivity and specificity of Wellens’ sign to predict LAD culprit were 24.6% and 96.2%, respectively.
Keywords: Wellens’ sign; NSTEMI; LAD culprit
| Introduction | ▴Top |
Electrocardiogram plays a fundamental role in the diagnosis and risk-stratification in patients with acute myocardial infarction. Acute myocardial infarction due to a culprit lesion in the left anterior descending (LAD) artery, which supplies a large territory of the left ventricle, results in wide-spread myocardial injury leading to worse clinical outcomes [1, 2]. Therefore, it is crucial to promptly identify patients with LAD culprit lesion who are at an increased risk for adverse events.
Unlike in patients with ST-segment elevation myocardial infarction, the identification of the infarct-related artery is often challenging in non-ST-segment elevation acute coronary syndrome since the location of ST-segment changes generally does not correlate with infarct location. Nevertheless, the presence of either symmetrically inverted or biphasic T waves in anterior precordial leads, so-called Wellens’ sign, has been reported to predict LAD culprit lesion in patients with unstable angina in the studies published almost 3 decades ago when sensitive biomarker cardiac troponin was not available [3-5]. Although it is assumed that some of the patients with unstable angina in these studies would have had troponin elevation and been diagnosed with non-ST-elevation myocardial infarction (NSTEMI) if cardiac troponin had been available, the prevalence of Wellens’ sign and its predictive value for LAD culprit lesion have not been clarified in a contemporary NSTEMI cohort.
In this context, we aimed to evaluate the prevalence of Wellens’ sign and its predictive value for LAD culprit lesion in patients with NSTEMI who underwent coronary angiography.
| Materials and Methods | ▴Top |
We performed a retrospective analysis on 481 consecutive NSTEMI patients who underwent coronary angiography within 5 days from presentation between January 2013 and June 2014. Myocardial infarction was diagnosed in accordance with the European Society of Cardiology and American College of Cardiology criteria [6]. Inclusion criteria were: 1) Troponin I level greater than the 99th percentile reference value before cardiac catheterization; 2) Chest pain (or anginal equivalent) or ischemic change on electrocardiogram including horizontal or down-sloping ST-segment depression (≥ 0.05 mV) or T-wave inversion (≥ 0.10 mV) in two or more contiguous leads; and 3) The absence of ST-segment elevation on electrocardiogram. We excluded patients with complete bundle branch block (n = 49), those with ventricular paced rhythm (n = 8), those with electrocardiographic left ventricular hypertrophy (n = 99), and those with pathologic Q wave in leads V2 and V3 (n = 5). In addition, we excluded patients with history of coronary artery bypass grafting (CABG) (n = 31) since identification of the culprit lesion in those patients is often difficult. We also excluded patients with more than one presumed culprit lesions (n = 15).
The present study complied with the Declaration of Helsinki and was approved by the institutional review board.
Demographic, hemodynamic and laboratory data
Electronic medical records were reviewed and following patients’ demographic data such as age, gender, body mass index, history of hypertension, history of diabetes mellitus, history of hyperlipidemia, history of chronic kidney disease, personal and family history of coronary artery disease (CAD), current smoking status, and previous myocardial infarction were abstracted. Thrombolysis in myocardial infarction (TIMI) risk score was calculated and classified into three groups: low risk (0 - 2), intermediate risk (3 - 4), and high risk (5 - 7). Initial presenting vital signs such as systolic blood pressure, diastolic blood pressure, and heart rate were recorded. In addition, the presence of chest pain at emergency department was recorded.
Laboratory data on admission including white blood cell count, hemoglobin level, and estimated glomerular filtration rate (eGFR) were recorded. Cardiac troponin I was measured using the second-generation VITROS® troponin I assay (Ortho-Clinical Diagnostics Inc., NJ, USA). The upper limit of normal for cardiac troponin I was 0.034 µg/L, which represented the 99th percentile reference value.
Transthoracic echocardiography was performed in a standard manner during hospitalization. Left ventricular ejection fraction was obtained using either the Teichholz or biplane Simpson’s method.
Electrocardiogram
Standard 12-lead electrocardiograms (25 mm/s and 10 mm = 1 mV) were obtained from all patients at the time of presentation to the emergency department and were reviewed by two independent reviewers in a blinded fashion. In the events of any discrepancy in the assessments, the two reviewers reached a consensus through discussion.
Wellens’ sign was present when there are either symmetrically inverted T waves (≥ 0.10 mV) or biphasic T waves in both leads V2 and V3 along with previous studies [3, 4]. Example electrocardiograms with symmetrically inverted T waves (≥ 0.10 mV) (Fig. 1a) and biphasic T waves in both leads V2 and V3 (Fig. 1b) are presented in Figure 1. In patients with symmetrically inverted T waves, a depth of T-wave inversion was measured from T-P segment as the baseline. ST-segment deviations were measured at the J point. ST-segment depression ≥ 0.05 mV in more than two contiguous leads was recorded. The cut-off of ≥ 0.05 mV was chosen in line with current universal definition of myocardial infarction [6]. The location of ST-segment depression was recorded as anterior (V1 - V4), lateral (I, aVL,V5, and V6), and inferior (II, III, and aVF).
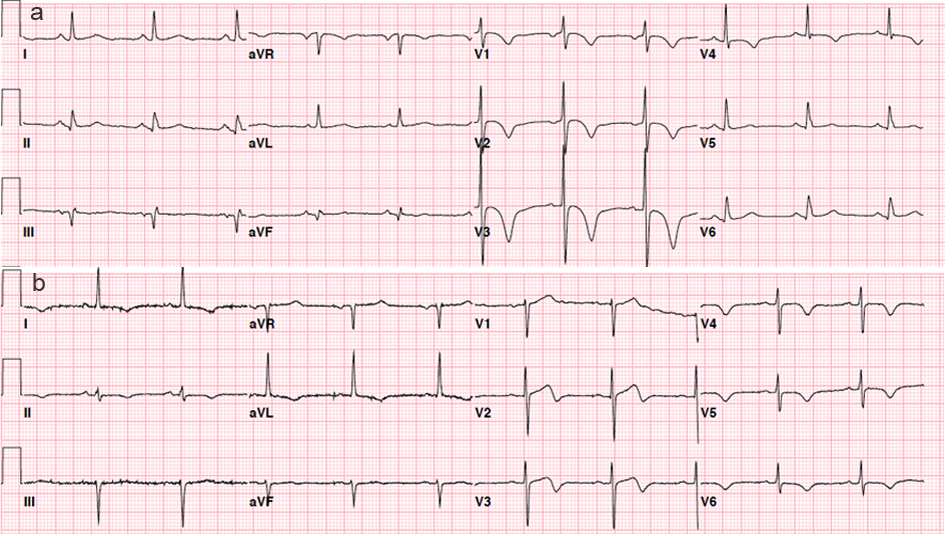 Click for large image | Figure 1. Electrocardiograms with symmetrically inverted T waves (≥ 0.10 mV) (Fig. 1a) and biphasic T waves in both leads V2 and V3 (Fig. 1b). |
Left ventricular hypertrophy defined by Sokolow-Lyon voltage amplitude criteria SV1 + RV5 or V6 ≥ 3.5 mV and Cornell voltage criteria SV3 + RaVL ≥ 2.8 mV in men and ≥ 2.0 mV in women [7, 8]. In patients with left anterior hemi-block, left ventricular hypertrophy was defined by SIII + maximal precordial R + S ≥ 3.0 mV [9].
Coronary angiography
All patients underwent coronary angiography within 5 days from presentation. An independent cardiologist blinded to the clinical data reviewed all coronary angiography, and the assessment was compared to the primary assessment by the treating cardiologist. In the event of a discrepancy between the assessments, a third investigator made the final interpretation. Obstructive CAD was defined as stenosis ≥ 50% in the left main coronary artery and ≥ 70% in any other epicardial coronary arteries. The culprit lesion was determined based on the echocardiographic and angiographic findings including previous coronary angiography, if available. Proximal LAD artery was defined as that proximal to the first septal branch. Revascularization procedures including percutaneous coronary intervention (PCI) and CABG were performed at the discretion of the treating physician. In addition, coronary blood flow was graded according to TIMI criteria [10].
End points
The primary end point was the prevalence of LAD culprit. In addition, in-hospital mortality and recurrent myocardial infarction were recorded.
Statistic analyses
Data was expressed as either a number (percentage) or median (interquartile range). Continuous variables were compared using the Wilcoxon rank sum test. Dichotomous variables were compared using the Chi-square test or Fisher’s exact test. Two-sided P values < 0.05 were considered statistically significant. All statistical analyses were performed using R software (version 3.0.1).
| Results | ▴Top |
Baseline, hemodynamic, and laboratory data
After excluding 207 patients who met the exclusion criteria, a total of 274 patients with NSTEMI who underwent coronary angiography were included, of whom 24 patients (8.8%) had Wellens’ sign. Baseline demographic and clinical characteristics are summarized in Table 1. Baseline clinical characteristics are comparable between patients with or without Wellens’ sign except for that patients with Wellens’ sign were more likely to be female. Majority of patients with or without Wellens’ sign had chest pain at emergency department. Patients with Wellens’ sign had a lower left ventricular ejection fraction than those without it, whereas peak troponin I values were comparable between the two groups.
 Click to view | Table 1. Demographic, Hemodynamic, and Laboratory Characteristics of Patients With and Without Wellens’ Sign |
Electrocardiographic characteristics
Electrocardiographic characteristics are summarized and presented in Table 2. Among the 24 patients with Wellens’ sign, 10 patients had biphasic T waves in both leads V2 and V3, and 14 patients had symmetrically inverted T waves (≥ 0.10 mV) in both leads V2 and V3. Among the 14 patients with symmetric T-wave inversion, 11 had T-wave inversion (≥ 0.20 mV) and four had T-wave inversion (≥ 0.30 mV) in both leads V2 and V3. Among the 24 patients with Wellens’ sign, 14 patients had similar T-wave abnormalities in lead V1, 18 patients in lead V4, 12 patients in lead V5, and eight patients in lead V6. Patients with Wellens’ sign were less likely to have concomitant ST depression.
 Click to view | Table 2. Electrocardiographic Characteristics of Patients With and Without Wellens’ Sign |
The sensitivity and specificity of electrocardiographic findings predicting for LAD culprit are presented in Table 3. Wellen’s sign (presence of either symmetrically inverted T waves (≥ 0.10 mV) or biphasic T waves in both leads V2 and V3) had a sensitivity of 24.6% and a specificity of 96.2% for LAD culprit lesion. Biphasic T waves in both leads V2 and V3 had a sensitivity of 12.3% and a specificity of 99.0%. Symmetric T-wave inversion (≥ 0.10 mV) in both leads V2 and V3 had a sensitivity of 12.3% and a specificity of 97.1%. As the cut-off of T-wave inversion increases, a sensitivity fell to 9.2% for T-wave inversion (≥ 0.20 mV) and 3.1% for T-wave inversion (≥ 0.30 mV) with specificity of 97.6% for T-wave inversion (≥ 0.20 mV) and 99.0% for T-wave inversion (≥ 0.30 mV), respectively.
 Click to view | Table 3. Predictive Values of Wellens’ Sign, Either Symmetrically Inverted T Waves (≥ 0.10 mV) or Biphasic T Waves in Both Leads V2 and V3, for Left Anterior Descending Artery Culprit |
Angiographic characteristics and revascularization procedures
Angiographic characteristics are presented in Table 4. Among the 24 patients with Wellens’ sign, 16 patients had a LAD culprit (eight proximal and eight mid LAD), two had a non-LAD culprit (circumflex lesions), and six had non-obstructive CAD. Patients with Wellens’ sign were more likely to have LAD culprit compared to those without it (66.7% vs. 19.6%, P < 0.001). Among the 16 patients with Wellens’ sign and LAD culprit, one patient had 100% occlusion, seven patients had 99% stenosis, five patients had 90% stenosis, and three patients had 75% stenosis. Patients with Wellens’ sign were less likely to have pre-procedural TIMI flow 0/1 compared with those without it (4.2% vs. 20.0%, P = 0.058). Patients with Wellens’ sign were less likely to have left main and/or three-vessel disease (4.2% vs. 20.4%, P = 0.057). The rate of non-obstructive CAD was comparable between the two groups (25.0% vs. 21.6%, P = 0.70).
 Click to view | Table 4. Angiographic Characteristics and In-Hospital Clinical Outcomes of Patients With and Without Wellens’ Sign |
The rates of revascularization procedures such as PCI and CABG are comparable between patients with or without Wellens’ sign. Except for one patient with three-vessel disease who underwent CABG, all of the patients with Wellens’ sign and LAD culprit received PCI to LAD during the index admission.
Endpoints
In-hospital clinical outcomes are summarized in Table 4. In our cohort of patients with NSTEMI, one patient died during the index admission and one patient suffered from recurrent myocardial infarction. The rate of in-hospital mortality and recurrent myocardial infarction were similar between the two groups.
| Discussion | ▴Top |
Our study revealed that: 1) Wellens’ sign, defined as the presence of either symmetrically inverted T waves (≥ 0.10 mV) or biphasic T waves in both leads V2 and V3, was seen in 8.8% of the patients with NSTEMI; 2) Two-thirds of the NSTEMI patients with Wellens’ sign had LAD culprit lesion and one-third had proximal LAD culprit lesion; and 3) Sensitivity and specificity of Wellens’ sign to predict LAD culprit lesion were 24.6% and 96.2%, respectively.
More than 3 decades ago, de Zwaan et al were the first to describe symmetrically inverted or biphasic T waves in precordial leads in patients with unstable angina, which later became to be known as Wellens’ sign [3]. In their study, 17.9% of the patients with unstable angina had this electrocardiographic finding. Among the patients with Wellens’ sign who underwent coronary angiography, 69.2% of them had either total occlusion or high-grade stenosis in proximal LAD. During the index admission, 75.0% of the patients with Wellens’ sign who did not undergo coronary angiography developed extensive anterior wall myocardial infarction. Subsequently, de Zwaan et al re-evaluated Wellens’ sign in a large cohort of 1,260 patients with unstable angina and reported the prevalence of Wellens’ sign as 16.2% [4]. Coronary angiography was performed in 88.2% of the patients with Wellens’ sign and 29% of the patients with Wellens’ sign had either total occlusion or high-grade stenosis of proximal LAD. Among patients with Wellens’ sign who did not undergo early revascularization, 30.3% of them developed acute myocardial infarction during follow-up, suggesting that Wellens’ sign identifies high-risk patients with impending LAD occlusion [3, 4].
These pioneer studies were performed before the wide-spread use of sensitive biomarker cardiac troponin [5] and it can be assumed that some of the unstable angina patients in these studies would have been diagnosed with NSTEMI if cardiac troponin had been available. The clinical implication of Wellens’ sign in a contemporary NSTEMI cohort has not been evaluated. Our study revealed that Wellens’ sign was seen in 8.8% of NSTEMI patients and that 66.7% of the patients with Wellens’ sign had LAD culprit lesion and 33.3% of those had proximal LAD culprit, which was consistent with the result of prior study [4]. The presence of Wellens’ sign had a predictive value for LAD culprit with a sensitivity of 24.6% and a specificity of 96.2%. Notably, despite its high specificity of Wellens’ sign for LAD lesion, it was also observed in patients with non-LAD culprit lesions and non-obstructive CAD. It has been reported that Wellens’ sign can be seen in a patient with normal coronary artery, mitral valve prolapse, and Prinzmetal’s angina [3].
Although the exact mechanism of Wellens’ sign has not been fully understood, a probable explanation is a brief transient episode of myocardial ischemia [4]. It has been reported that, during an attack of chest pain, patients with Wellens’ sign lose their characteristic T-wave abnormalities or develop ST-segment elevation [4]. In our present study, almost all patients with Wellens’ sign had pre-procedural TIMI flow 2 or 3. This suggests that Wellens’ sign represents impending but canalized LAD culprit lesion in patients with NSTEMI.
In our present study, we chose cut-off of ≥ 0.10 mV for symmetrically inverted T waves. This is because as the cut-off of T-wave inversion increases, a sensitivity for LAD culprit falls to 9.2% for T-wave inversion (≥ 0.20 mV) and 3.1% for T-wave inversion (≥ 0.30 mV) with a similar specificity of 97.6% for T-wave inversion (≥ 0.20 mV) and 99.0% for T-wave inversion (≥ 0.30 mV). In addition, it was reported that the depth of inverted T waves did not carry a prognostic significance in de Zwaan’ study [3].
This study has several limitations, including a retrospective design, a relatively small number of patients, and the lack of data on long-term clinical outcomes. We only included patients who underwent coronary angiography, and thus generalizability of our findings is limited. In addition, since follow-up electrocardiograms were available in a limited number of patients, we do not know if Wellens’ sign improved after revascularization. However, prior studies demonstrated the resolution of those T-wave abnormalities after revascularization [3, 4]. Finally, the prognostic value of Wellens’ sign for predicting short- and long-term outcomes is subject to further studies in larger cohorts.
Conclusions
The present study demonstrated that Wellens’ sign was seen in 8.8% patients with NSTEMI. Two-thirds of the NSTEMI patients with Wellens’ sign had LAD culprit lesion and one-third had proximal LAD culprit lesion. Wellens’ sign had a predictive value for LAD culprit with a sensitivity of 24.6% and a specificity of 96.2%.
Acknowledgments
None.
Financial Disclosure
None.
Conflict of Interest
None.
Informed Consent
Not applicable.
Author Contributions
All the authors contributed to the institutional review board application process, data collection, analysis of the data, and writing of the manuscript.
| References | ▴Top |
- Haim M, Hod H, Reisin L, Kornowski R, Reicher-Reiss H, Goldbourt U, Boyko V, et al. Comparison of short- and long-term prognosis in patients with anterior wall versus inferior or lateral wall non-Q-wave acute myocardial infarction. Secondary Prevention Reinfarction Israeli Nifedipine Trial (SPRINT) Study Group. Am J Cardiol. 1997;79(6):717-721.
doi - Behar S, Rabinowitz B, Zion M, Reicher-Reiss H, Kaplinsky E, Abinader E, Agmon J, et al. Immediate and long-term prognostic significance of a first anterior versus first inferior wall Q-wave acute myocardial infarction. Secondary Prevention Reinfarction Israeli Nifedipine Trial (SPRINT) Study Group. Am J Cardiol. 1993;72(18):1366-1370.
doi - de Zwaan C, Bar FW, Wellens HJ. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J. 1982;103(4 Pt 2):730-736.
doi - de Zwaan C, Bar FW, Janssen JH, Cheriex EC, Dassen WR, Brugada P, Penn OC, et al. Angiographic and clinical characteristics of patients with unstable angina showing an ECG pattern indicating critical narrowing of the proximal LAD coronary artery. Am Heart J. 1989;117(3):657-665.
doi - Hamm CW, Ravkilde J, Gerhardt W, Jorgensen P, Peheim E, Ljungdahl L, Goldmann B, et al. The prognostic value of serum troponin T in unstable angina. N Engl J Med. 1992;327(3):146-150.
doi pubmed - Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESCAAHAWHFTFfUDoMI, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60(16):1581-1598.
doi pubmed - Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37(2):161-186.
doi - Casale PN, Devereux RB, Kligfield P, Eisenberg RR, Miller DH, Chaudhary BS, Phillips MC. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6(3):572-580.
doi - Gertsch M, Theler A, Foglia E. Electrocardiographic detection of left ventricular hypertrophy in the presence of left anterior fascicular block. Am J Cardiol. 1988;61(13):1098-1101.
doi - Timi Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. N Engl J Med. 1985;312(14):932-936.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


