| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website http://www.cardiologyres.org |
Review
Volume 11, Number 1, February 2020, pages 9-14
Kawasaki Disease: Global Burden and Genetic Background
Karim Elakabawia, b, e, Jing Linc, e, Fuyong Jiaod, Ning Guoa, f, Zuyi Yuana, f
aCardiovascular Department, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi 710061, China
bCardiovascular Department, Benha University, Benha 13518, Egypt
cDepartment of Child and Adolescent Health Science Center, School of Public Health, Xi’an Jiaotong University, Xi’an, Shaanxi 710061, China
dChildren’s Hospital, Shaanxi Provincial People’s Hospital, Xi’an, Shaanxi 710061, China
eThese two authors contributed equally.
fCorresponding Author: Zuyi Yuan, Cardiovascular Department, First Affiliated Hospital of Xi’an Jiaotong University, 277 West Yanta Road, Xi’an, Shaanxi 710061, China; Ning Guo, Cardiovascular Department, First Affiliated Hospital of Xi’an Jiaotong University, 277 West Yanta Road, Xi’an, Shaanxi 710061, China
Manuscript submitted November 20, 2019, accepted December 5, 2019
Short title: KD: Global Burden and Genetic Background
doi: https://doi.org/10.14740/cr993
| Abstract | ▴Top |
Kawasaki disease (KD) is a childhood vasculitides associated with serious coronary artery lesions. It is the most common cause of pediatric acquired heart disease in developed countries, and is increasingly reported from many rapidly industrializing developing countries. The incidence varies widely among different nations and is highest in North-East Asian countries, where almost 1 in 100 children in Japan having the disease by age of 5, where the lowest incidence reported in sub-Saharan Africa. The etiology of KD is still uncertain; interaction between a genetic predisposition and several environmental and immunological factors has been hypothesized. Several susceptibility genes were identified to be associated with the development of KD and increased risk of coronary artery lesions. Gene-gene associations and alteration of deoxyribonucleic acid (DNA) methylation are also found to play key roles in the pathogenesis and prognosis of KD. This article will focus on the global epidemiological patterns of KD, and the currently known genetic predisposition.
Keywords: Kawasaki disease; Epidemiology; Genetics
| Introduction | ▴Top |
Kawasaki disease (KD), first reported by the Japanese physician Tomisaku Kawasaki, is considered the most common childhood vasculitides in developed countries [1, 2]. Various epidemiologic reports showed a significantly increasing incidence of KD in rapidly industrializing developing countries as well, such as China, India, and Latin American countries. This may be due to an actual increase in incident cases or the improvements in health facilities, and the widespread use of antimicrobials and vaccines that helped to eliminate infectious diseases with their similar fever and rash allowing more awareness and ascertainment of KD [2, 3].
The disease causes inflammatory changes to the vessel walls of small and medium-sized arteries of any area of the body. However, coronary arteries are predominantly involved, and coronary artery lesions can occur in up to 25% of untreated children with KD resulting in serious complications as coronary artery ectasia/dilatation, coronary artery aneurysm, and acute myocardial infarction [4]. Therefore, early detection and prompt treatment are crucial, since treatment with intravenous immunoglobulin (IVIG) within 10 days after disease onset can lower the incidence of aneurysm to < 5% [5, 6]. The peak age incidence of KD generally range between 6 months and 5 years, and usually presents with fever, skin rash, diffuse mucosal inflammation, non-exudative conjunctivitis, cervical lymphadenopathy and extremities changes [7, 8].
The etiology and pathogenesis of KD remain uncertain. The presence of familial aggregation and the increased incidence by 10 times in Asian population suggest a strong genetic origin [9]. However, genetic factors alone cannot explain seasonal variations or geographic and temporal clustering of KD cases [10]. Additional factors, such as infectious agents (bacteria, viruses, fungi, etc) [11], environmental rigger or autoimmune reactions [12] are needed for the onset of the disease, but their definite role remains unclear. In addition, epidemics of KD have been reported, most known in Japan in 1982 and 1986 [13]. Several studies have also documented a correlation between higher socioeconomic status, smaller family size, and urbanization and increased KD incidence [14, 15].
Since 1980s, KD attained a significant public health concern. As with the control of infection, availability of antimicrobial agents, and enhanced general hygiene; the incidence of rheumatic fever and other infectious diseases declined in developed countries. Meanwhile the incidence of KD remarkably increased and became the commonest cause of pediatric acquired heart disease, particularly in Japan, Korea, the USA, and many other developed countries [16].
| Global Epidemiology | ▴Top |
KD has been documented in more than 60 countries and cross all ethnicities [4, 17] (Fig. 1, [2]). The incidence of KD is increasing in several countries especially rapidly industrializing nations, which may reflect a role of air pollution in the pathogenesis of KD [3]. The diagnosis of KD till now is based only on the characteristic clinical features with the presence of a proportion of incomplete or atypical KD cases that have no standard criteria for their diagnosis, which is usually achieved through clinical and laboratory data supported by expert opinion [4, 18]. This difficulty in diagnosis makes the true incidence and burden of the disease to be still unknown [2].
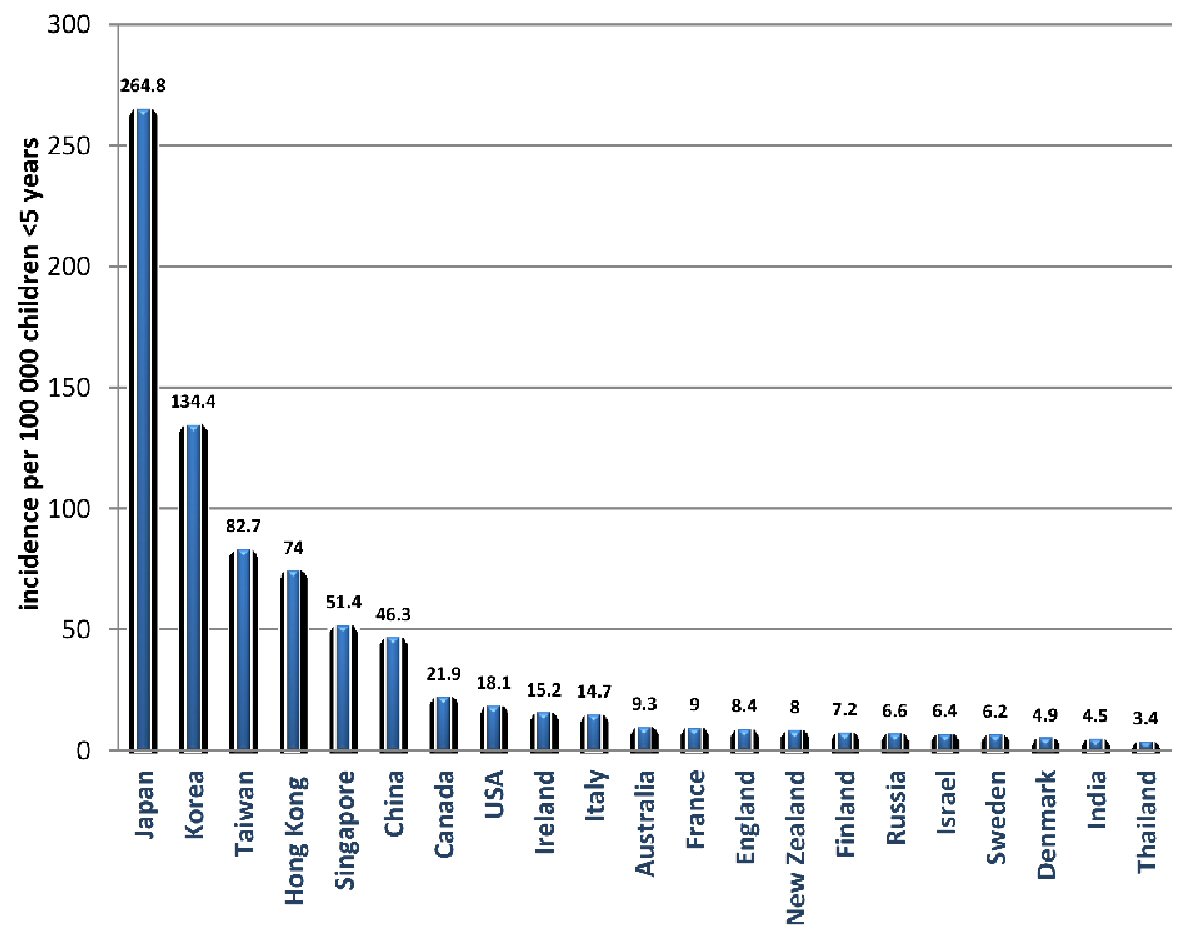 Click for large image | Figure 1. The global incidence of Kawasaki disease per 100,000 children under 5 years old [2]. |
Epidemiological distributions of KD
The incidence of KD varies greatly among different regions. In North America, Europe, and Australia, the incidence of KD ranges from 5 - 22 per 100,000 children < 5 years old. In these countries the incidence was increasing, partly as a result of increased ascertainment, until the past 10 years, when the incidence becomes nearly constant [2, 17]. Conversely, North-Eastern Asian countries, especially Japan, Korea, and Taiwan report an incidence of more than 10 times greater than North America, Australia and Europe; and it continues to increase as reported during the last two decades [19-21]. In the rapidly growing economics like China and India, although the epidemiological reports are less accurate, the incidence appears to be significantly growing and follows the epidemiological patterns of the North-Eastern Asian countries [22, 23].
KD in North America, Europe and Australia
KD incidence in these countries is nearly constant with the lowest incidence in Scandinavian countries, and the highest incidence in North America [2, 17]. In USA, the incidence is estimated to be 17 - 21 per 100,000 children < 5 years old [17]. Both data from the USA and Canada showed significant ethnic variation, with a markedly higher rate among American Asians and peoples of the Pacific Islands supporting the genetic background of KD [17, 24]. Most of the European countries report an incidence rate < 16 per 100,000 children < 5 years old; there were also noticeable ethnic variations, with higher incidence reported in children of Eastern Asian descent [25]. In Australia, the incidence rate ranges between 8 and 10 per 100,000 children < 5 years, and it is not determined whether the incidence has plateaued or is still increasing [25].
KD in Japan, South Korea and Taiwan
The incidence rates of KD in these countries are the highest worldwide (> 50 per 100,000 children < 5 years old) and the number continues to increase [19-21]. The highest rate globally is reported in Japan with an estimate of 264 per 100,000 children < 5 years. Recurrence rate of KD was 3.5% of cases, mortality rate was < 0.02%, and resistance to IVIG reported in 17.0% of cases [21]. South Korea reported the second highest incidence rate (134.4 per 100,000 children < 5 years) [20]. Data on KD in Taiwan are extracted from the records of the national health insurance program with an estimated incidence of (82.8 per 100,000 children < 5 years) and reported coronary complications in 5.4% of the cases [21, 26].
KD in China
The incidence data of KD are available from studies conducted in different provinces, as an accurate nationwide study is difficult to conduct in the most populous country in the world. The reported incidence varies between 7.06 and 55.1 per 100,000 children < 5 years [27-29]. For example, in Beijing the incidence rate increased from 40.9 per 100,000 children in 2000 to 55 per 100,000 in 2004, and in Shanghai the rate increased 3 times in less than 20 years (from 16.8 per 100,000 in 1998 to 50.5 per 100,000 children in 2012). On the other hand, in the lower income Sichuan province, the reported incidence was 7 per 100,000 children, largely lower than other provinces which may reflect a lack of access to medical care services due to relative lower social status. In Hong Kong, the incidence rate is the highest in China (74 per 100,000 children < 5 years) [30]. Despite the shortage of a nationwide data in China, it appears that it follows the trend of the increasing incidence of KD similar to that of other East Asian countries.
KD in other Asian countries
A gradual increase in the incidence of KD has been reported in India, Thailand, and many other countries across Asia [23, 30]. The lack of nationwide epidemiological data makes it difficult to determine whether this is a true increase due to environmental and climate changes associated with industrialization or just an increase ascertainment resulting from more awareness by health care professionals and increased access to medical care following the rapid economic growth [23]. Before 1990, there were only three available reports of KD in India. After this time, KD has been reported in almost all regions of the country with incidence data of 4.5 cases per 100,000 children less than 15 years old [31].
KD in the Middle East and Sub-Saharan Africa
Except for some studies conducted in Turkey and Iran, limited data have been reported from other Middle Eastern countries [32, 33]. A reason of shortage of incidence reports from this region could be due to the lower socioeconomic status and a lack of census data for children < 5 years of age. A study from Egypt reported coronary aneurysms denoting missed childhood KD cases when they reviewed a series of 580 patients ≤ 40 years of age presenting with symptoms of coronary artery ischemia [34]. Also, Agha et al reported two cases with incomplete KD in children < 5 years old complicated with coronary aneurysm [35]. The data for KD incidence are lacking in sub-Saharan Africa, however several sporadic cases have been reported across many countries in the region [36]. These findings raise the possibility that KD is not that uncommon in the Middle East and sub-Saharan Africa as previously thought, and increased awareness and diagnosis may reveal the true incidence of KD.
KD in Latin America
Prior to the formation of the Latin American Kawasaki Disease Network (REKAMLATINA), which included 20 countries, little was known about the epidemiology and prevalence of KD in these countries [37]. In Chile as an example, there were several reports of increasing cases of KD, which also may be a result of the increased awareness and ascertainment of the disease [38].
Seasonality of KD
Many Asian countries such as Japan and South Korea had reported distinctive seasonal patterns of KD, where they reported a summer peak in July and a winter peak in January [19, 20]. In USA, Canada, Europe, and temperate regions of Australia, they reported peak of incidence of KD occurring in the winter season except for Hawaii, which has different seasonality [14-17]. It has been hypothesized that tropospheric wind patterns arising from north-eastern China are associated with KD peaks in Japan, Hawaii, and southern California suggesting that KD could be induced by an airborne pathogen arising from this area [39].
| Genetic Background | ▴Top |
Ethnicity significantly affects the genetic ability to develop KD. Not only as mentioned before that the incidence is increased by 10 times in Asian children [9], but also the genes associated with KD and their degree of expression seem to vary among different ethnicities [40]. In response to this situation, several susceptibility genes including single-nucleotide polymorphisms (SNP) for the ITPKC, CASP3, FCGR2A, BLK, ORAI, and CD40 genes have been recognized through genome-wide association and genome-wide linkage analysis employed in different ethnic populations to have an association with the etiology and prognosis of KD, and also with the development of coronary artery aneurysms (CAA) [41-43].
Gene-gene associations
Recently, several studies have indicated an important role of gene-gene interactions in KD pathogenesis, and have identified that varying gene-gene associations could predict the development of KD or the increased risk of its complications of CAA; and its prediction power is greater than that of individual SNPs [44-46]. Kuo and colleagues examined 384 SNPs for 159 immune-related candidate genes in of deoxyribonucleic acid (DNA) samples collected from 73 KD patients with CAA (73 patients), KD patients without CAA (153 patients), and 575 healthy controls. By logistic regression analysis, they identified that the combined acquisition of PDE2A gene (rs341058) and CYFIP2 gene (rs767007) significantly increased KD susceptibility (odds ratio (OR) = 3.54; P = 4.14 × 10-7), while the combined acquisition of LOC100133214 gene (rs2517892) and IL2RA gene (rs3118470) significantly increased the risk of CAA in KD patients (OR = 5.35; P < 0.001) [9].
In another study conducted by Kuo et al, they noticed that high-risk genotype patients with combined acquisition of ITPKC gene (rs28493229) and CASP3 gene (rs113420705) were more associated with CAA formation (P = 0.0227, OR = 3.06) and had a higher IVIG resistance rate in comparison to patients with individual susceptible SNP [47].
Gene-expression patterns associated with KD
During the past years, gene expression signatures have generated new clues for the diagnosis and pathogenesis of various infectious and inflammatory diseases with unclear etiology [48, 49]. According to the current guidelines [4, 18], the diagnosis of KD relies on clinical features that are usually similar to other infectious or inflammatory diseases, leading to delayed or missed diagnosis and treatment in many cases with an increased liability to complications.
In a study performed by Wright et al [50], they presented a rapid diagnostic blood test dependent on the measurement of small numbers of host gene transcripts, which would help an early diagnosis of KD and definite differentiation from other infectious or inflammatory diseases. They recognized a 13-transcript gene expression signature with high sensitivity and specificity for KD that included eight genes (CACNA1E, DDIAS, KLHL2, PYROXD2, SMOX, ZNF185, LINC02035, and CLIC3) with an increased expression in KD relative to other conditions, and five other genes (S100P, IFI27, HS.553068, CD163, and RTN1) that showed a decreased expression in KD. These findings, supported by other earlier studies [51, 52], might form the basis of a fast and easy applicable diagnostic laboratory test for KD.
DNA methyltransferases expression in KD
Methylation is the principal epigenetic modification in mammals’ genome, and in general, the DNA methylation alteration of CpG islands present in the promoter region of the genes can lead to a powerful transcription inhibition [53]. DNA methylation is controlled by two groups of enzymes, the first are DNA methyltransferases (DNMTs) and the second are the Ten-eleven translocation (TET) family [53, 54].
In 2018 Chen et al [55] compared the CpG methylation status in KD patients and controls, and found that most of CpG loci (97%) revealed hypomethylation with only 3% showed hypermethylation; a finding suggesting that the majority of genes in KD patients have a hypomethylation status which results in an over-expression of these genes with increased activity of varies immune mechanisms including T helper 1 (Th1), Th2, Th17, innate immunity, acquired immunity, cytokines, etc. The exact reason why most genes are activated remains uncertain. DNA hypomethylation by imbalanced DNMTs and TET activities is hypothesized to be a key factor in the pathogenesis of this condition.
In another study Huang [56] et al identified differential expressions of DNMTs and TETs’ mRNA levels in KD patients when compared to both febrile and healthy controls, with a significant decrease in the mRNA levels of DNMT1 and DNMT3A, and a significant increase in TET2 levels in KD patients. In addition, following an IVIG treatment, they noted a decrease in the mRNA level of TET2 and significantly lower DMNT1 mRNA levels between patients with CAA and those without. These studies emphasize the hypothesis of altered DNA methylation to be among the first pathogenic changes during the acute phase of KD.
| Conclusions | ▴Top |
KD is an acute febrile vasculitic disease in children and is the most common cause of acquired heart disease in children and young adults in developed counties. It is also increasingly reported in several developing countries, including China, India, Middle East and Latin American countries. The etiology of KD is still unclear; interaction between a genetic predisposition and several environmental and immunological factors has been hypothesized. Several susceptibility genes were recognized to have an association with the development of KD and an increased predisposition to its compilations. Gene-gene interactions and alteration of DNA methylation are also found to play key roles in the pathogenesis and prognosis of KD.
Acknowledgments
The authors acknowledge the support of the China Scholarship Council (CSC).
Financial Disclosure
This work was supported by grants from the National Natural Science Foundation of China, the Fundamental Research Funds for the Central Universities, and the First Affiliated Hospital of XJTU Fund.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Author Contributions
Karim Elakabawi and Fuyong Jiao contributed to the conception and design of the work; Karim Elakabawi and Jing Lin contributed to the literature search, and drafting the manuscript; Fuyong Jiao, Ning Guo, and Zuyi Yuan critically revised the manuscript.
| References | ▴Top |
- Kawasaki T. [Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children]. Arerugi. 1967;16(3):178-222.
- Lin MT, Wu MH. The global epidemiology of Kawasaki disease: Review and future perspectives. Glob Cardiol Sci Pract. 2017;2017(3):e201720.
doi pubmed - Jiao F, Jindal AK, Pandiarajan V, Khubchandani R, Kamath N, Sabui T, Mondal R, et al. The emergence of Kawasaki disease in India and China. Glob Cardiol Sci Pract. 2017;2017(3):e201721.
doi pubmed - Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on rheumatic fever, endocarditis and Kawasaki disease, council on cardiovascular disease in the Young, American Heart Association. Circulation. 2004;110(17):2747-2771.
doi pubmed - McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927-e999.
doi pubmed - Newburger JW, Takahashi M, Burns JC. Kawasaki Disease. J Am Coll Cardiol. 2016;67(14):1738-1749.
doi pubmed - Gatterre P, Oualha M, Dupic L, Iserin F, Bodemer C, Lesage F, Hubert P. Kawasaki disease: an unexpected etiology of shock and multiple organ dysfunction syndrome. Intensive Care Med. 2012;38(5):872-878.
doi pubmed - Minich LL, Sleeper LA, Atz AM, McCrindle BW, Lu M, Colan SD, Printz BF, et al. Delayed diagnosis of Kawasaki disease: what are the risk factors? Pediatrics. 2007;120(6):e1434-1440.
doi pubmed - Kuo HC, Li SC, Guo MM, Huang YH, Yu HR, Huang FC, Jiao F, et al. Genome-wide association study identifies novel susceptibility genes associated with coronary artery aneurysm formation in Kawasaki disease. PLoS One. 2016;11(5):e0154943.
doi pubmed - Burgner D, Harnden A. Kawasaki disease: what is the epidemiology telling us about the etiology? Int J Infect Dis. 2005;9(4):185-194.
doi pubmed - Chang LY, Lu CY, Shao PL, Lee PI, Lin MT, Fan TY, Cheng AL, et al. Viral infections associated with Kawasaki disease. J Formos Med Assoc. 2014;113(3):148-154.
doi pubmed - Guo MM, Tseng WN, Ko CH, Pan HM, Hsieh KS, Kuo HC. Th17- and Treg-related cytokine and mRNA expression are associated with acute and resolving Kawasaki disease. Allergy. 2015;70(3):310-318.
doi pubmed - Nakamura Y, Yashiro M, Uehara R, Sadakane A, Tsuboi S, Aoyama Y, Kotani K, et al. Epidemiologic features of Kawasaki disease in Japan: results of the 2009-2010 nationwide survey. J Epidemiol. 2012;22(3):216-221.
doi pubmed - Bell DM, Brink EW, Nitzkin JL, Hall CB, Wulff H, Berkowitz ID, Feorino PM, et al. Kawasaki syndrome: description of two outbreaks in the United States. N Engl J Med. 1981;304(26):1568-1575.
doi pubmed - Dean AG, Melish ME, Hicks R, Palumbo NE. An epidemic of Kawasaki syndrome in Hawaii. J Pediatr. 1982;100(4):552-557.
doi - Veasy LG, Wiedmeier SE, Orsmond GS, Ruttenberg HD, Boucek MM, Roth SJ, Tait VF, et al. Resurgence of acute rheumatic fever in the intermountain area of the United States. N Engl J Med. 1987;316(8):421-427.
doi pubmed - Uehara R, Belay ED. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol. 2012;22(2):79-85.
doi pubmed - Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, Kiyosawa N, Ishii M, et al. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Pediatr Int. 2005;47(2):232-234.
doi pubmed - Makino N, Nakamura Y, Yashiro M, Ae R, Tsuboi S, Aoyama Y, Kojo T, et al. Descriptive epidemiology of Kawasaki disease in Japan, 2011-2012: from the results of the 22nd nationwide survey. J Epidemiol. 2015;25(3):239-245.
doi pubmed - Kim GB, Han JW, Park YW, Song MS, Hong YM, Cha SH, Kim DS, et al. Epidemiologic features of Kawasaki disease in South Korea: data from nationwide survey, 2009-2011. Pediatr Infect Dis J. 2014;33(1):24-27.
doi pubmed - Huang WC, Huang LM, Chang IS, Chang LY, Chiang BL, Chen PJ, Wu MH, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003-2006. Pediatrics. 2009;123(3):e401-405.
doi pubmed - Du ZD, Zhang T, Liang L, Meng X, Li T, Kawasaki T, Nakamura Y, et al. Epidemiologic picture of Kawasaki disease in Beijing from 1995 through 1999. Pediatr Infect Dis J. 2002;21(2):103-107.
doi pubmed - Singh S, Aulakh R, Bhalla AK, Suri D, Manojkumar R, Narula N, Burns JC. Is Kawasaki disease incidence rising in Chandigarh, North India? Arch Dis Child. 2011;96(2):137-140.
doi pubmed - Lin YT, Manlhiot C, Ching JC, Han RK, Nield LE, Dillenburg R, Pepelassis D, et al. Repeated systematic surveillance of Kawasaki disease in Ontario from 1995 to 2006. Pediatr Int. 2010;52(5):699-706.
doi pubmed - Saundankar J, Yim D, Itotoh B, Payne R, Maslin K, Jape G, Ramsay J, et al. The epidemiology and clinical features of Kawasaki disease in Australia. Pediatrics. 2014;133(4):e1009-1014.
doi pubmed - Wu MH, Chen HC, Yeh SJ, Lin MT, Huang SC, Huang SK. Prevalence and the long-term coronary risks of patients with Kawasaki disease in a general population <40 years: a national database study. Circ Cardiovasc Qual Outcomes. 2012;5(4):566-570.
doi pubmed - Du ZD, Zhao D, Du J, Zhang YL, Lin Y, Liu C, Zhang T, et al. Epidemiologic study on Kawasaki disease in Beijing from 2000 through 2004. Pediatr Infect Dis J. 2007;26(5):449-451.
doi pubmed - Li XH, Li XJ, Li H, Xu M, Zhou M. Epidemiological survey of Kawasaki disease in Sichuan province of China. J Trop Pediatr. 2008;54(2):133-136.
doi pubmed - Ma XJ, Yu CY, Huang M, Chen SB, Huang MR, Huang GY, Shanghai Kawasaki Research G. Epidemiologic features of Kawasaki disease in Shanghai from 2003 through 2007. Chin Med J (Engl). 2010;123(19):2629-2634.
- Abstracts of the 10th International Kawasaki Disease Symposium. February 7-10, 2012. Kyoto, Japan. Pediatr Int. 2012;(Suppl 1):38-142.
- Singh S, Sharma A, Jiao F. Kawasaki disease: issues in diagnosis and treatment - a developing country perspective. Indian J Pediatr. 2016;83(2):140-145.
doi pubmed - Asadi-Pooya AA, Borzoee M, Amoozgar H. The experience with 113 patients with Kawasaki disease in Fars Province, Iran. Turk J Pediatr. 2006;48(2):109-114.
- Ozdemir H, Ciftci E, Tapisiz A, Ince E, Tutar E, Atalay S, Dogru U. Clinical and epidemiological characteristics of children with Kawasaki disease in Turkey. J Trop Pediatr. 2010;56(4):260-262.
doi pubmed - Rizk SR, El Said G, Daniels LB, Burns JC, El Said H, Sorour KA, Gharib S, et al. Acute myocardial ischemia in adults secondary to missed Kawasaki disease in childhood. Am J Cardiol. 2015;115(4):423-427.
doi pubmed - Agha HM, Hamza HS. Incomplete Kawasaki disease in Egypt. Glob Cardiol Sci Pract. 2017;2017(3):e201724.
doi pubmed - Badoe EV, Neequaye J, Oliver-Commey JO, Amoah J, Osafo A, Aryee I, Nyarko MY. Kawasaki disease in ghana: case reports from Korle Bu teaching hospital. Ghana Med J. 2011;45(1):38-42.
doi pubmed - Ulloa-Gutierrez R, Salgado AP, Tremoulet AH. Kawasaki disease in Latin American children: past, current, and future challenges. J Pediatric Infect Dis Soc. 2014;3(4):280-281.
doi pubmed - Borzutzky A, Hoyos-Bachiloglu R, Cerda J, Talesnik E. Rising hospitalization rates of Kawasaki Disease in Chile between 2001 and 2007. Rheumatol Int. 2012;32(8):2491-2495.
doi pubmed - Rodo X, Curcoll R, Robinson M, Ballester J, Burns JC, Cayan DR, Lipkin WI, et al. Tropospheric winds from northeastern China carry the etiologic agent of Kawasaki disease from its source to Japan. Proc Natl Acad Sci U S A. 2014;111(22):7952-7957.
doi pubmed - Onouchi Y. Genetics of Kawasaki disease: what we know and don't know. Circ J. 2012;76(7):1581-1586.
doi pubmed - Onouchi Y, Ozaki K, Burns JC, Shimizu C, Terai M, Hamada H, Honda T, et al. A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat Genet. 2012;44(5):517-521.
doi pubmed - Thiha K, Mashimo Y, Suzuki H, Hamada H, Hata A, Hara T, Tanaka T, et al. Investigation of novel variations of ORAI1 gene and their association with Kawasaki disease. J Hum Genet. 2019;64(6):511-519.
doi pubmed - Yamamoto-Shimojima K, Imaizumi T, Aoki Y, Inoue K, Kaname T, Okuno Y, Muramatsu H, et al. Elucidation of the pathogenic mechanism and potential treatment strategy for a female patient with spastic paraplegia derived from a single-nucleotide deletion in PLP1. J Hum Genet. 2019;64(7):665-671.
doi pubmed - Kim JJ, Hong YM, Sohn S, Jang GY, Ha KS, Yun SW, Han MK, et al. A genome-wide association analysis reveals 1p31 and 2p13.3 as susceptibility loci for Kawasaki disease. Hum Genet. 2011;129(5):487-495.
doi pubmed - Tsai FJ, Lee YC, Chang JS, Huang LM, Huang FY, Chiu NC, Chen MR, et al. Identification of novel susceptibility Loci for kawasaki disease in a Han chinese population by a genome-wide association study. PLoS One. 2011;6(2):e16853.
doi pubmed - Onouchi Y, Suzuki Y, Suzuki H, Terai M, Yasukawa K, Hamada H, Suenaga T, et al. ITPKC and CASP3 polymorphisms and risks for IVIG unresponsiveness and coronary artery lesion formation in Kawasaki disease. Pharmacogenomics J. 2013;13(1):52-59.
doi pubmed - Kuo HC, Hsu YW, Wu CM, Chen SH, Hung KS, Chang WP, Yang KD, et al. A replication study for association of ITPKC and CASP3 two-locus analysis in IVIG unresponsiveness and coronary artery lesion in Kawasaki disease. PLoS One. 2013;8(7):e69685.
doi pubmed - Anderson ST, Kaforou M, Brent AJ, Wright VJ, Banwell CM, Chagaluka G, Crampin AC, et al. Diagnosis of childhood tuberculosis and host RNA expression in Africa. N Engl J Med. 2014;370(18):1712-1723.
doi pubmed - Herberg JA, Kaforou M, Wright VJ, Shailes H, Eleftherohorinou H, Hoggart CJ, Cebey-Lopez M, et al. Diagnostic Test Accuracy of a 2-Transcript Host RNA Signature for Discriminating Bacterial vs Viral Infection in Febrile Children. JAMA. 2016;316(8):835-845.
doi pubmed - Wright VJ, Herberg JA, Kaforou M, et al. Diagnosis of kawasaki disease using a minimal whole-blood gene expression signature. JAMA Pediatr 2018;172:1-10.
doi pubmed - Hoang LT, Shimizu C, Ling L, Naim AN, Khor CC, Tremoulet AH, Wright V, et al. Global gene expression profiling identifies new therapeutic targets in acute Kawasaki disease. Genome Med. 2014;6(11):541.
doi pubmed - Popper SJ, Watson VE, Shimizu C, Kanegaye JT, Burns JC, Relman DA. Gene transcript abundance profiles distinguish Kawasaki disease from adenovirus infection. J Infect Dis. 2009;200(4):657-666.
doi pubmed - Shilatifard A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem. 2006;75:243-269.
doi pubmed - Liu Y, Peng W, Qu K, Lin X, Zeng Z, Chen J, Wei D, et al. TET2: a novel epigenetic regulator and potential intervention target for atherosclerosis. DNA Cell Biol. 2018;37(6):517-523.
doi pubmed - Chen KD, Huang YH, Ming-Huey Guo M, Lin TY, Weng WT, Yang HJ, Yang KD, et al. The human blood DNA methylome identifies crucial role of beta-catenin in the pathogenesis of Kawasaki disease. Oncotarget. 2018;9(47):28337-28350.
doi pubmed - Huang YH, Chen KD, Lo MH, Cai XY, Chang LS, Kuo YH, Huang WD, et al. Decreased DNA methyltransferases expression is associated with coronary artery lesion formation in Kawasaki disease. Int J Med Sci. 2019;16(4):576-582.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


