| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 11, Number 6, December 2020, pages 370-375
A Novel Non-Invasive Assessment of Cardiac Hemodynamics in Patients With Heart Failure and Atrial Fibrillation
Fadi Khraima, d, Mohammad Alhamaydehb, Ziad Faramandc, Samir Sabac, Salah Al-Zaitic
aUniversity of Calgary, Doha, Qatar
bConemaugh Memorial Medical Center, Johnstown PA, USA
cUniversity of Pittsburgh, Pittsburgh PA, USA
dCorresponding Author: Fadi Khraim, University of Calgary, Al-Rayyan Campus, P.O. Box 23133, Doha, Qatar
Manuscript submitted June 3, 2020, accepted September 23, 2020, published online November 2, 2020
Short title: Impedance Cardiography in Heart Failure
doi: https://doi.org/10.14740/cr1110
| Abstract | ▴Top |
Background: Heart failure (HF) and atrial fibrillation (AF) often coexist. The hemodynamic alterations induced by AF in patients with HF are well studied; however we lack reliable and non-invasive means to study these hemodynamic alterations in ambulatory patients. We sought to evaluate the clinical utility of impedance cardiography (ICG) as a novel and non-invasive tool to evaluate cardiac hemodynamics in ambulatory patients with HF and AF.
Methods: This was a single-center observational study. A convenient sample of ambulatory patients with chronic HF underwent non-invasive electrocardiogram (ECG) and hemodynamic monitoring using BioZ Dx impedance cardiographer. Hemodynamics were automatically computed and ECG data were interpreted by an independent reviewer.
Results: A total of 32 patients (62 ± 14 years of age; 66% male; ejection fraction 33±13%) were enrolled. There were no baseline demographic or clinical differences between those with AF (28%) and those without AF (72%). However, patients with AF exhibited lower stroke volume (60 ± 7 vs. 89 ± 29, P = 0.008), left ventricular work (33 ± 9 vs. 45 ± 13, P = 0.016), cardiac contractility (30 ± 8 vs. 40 ± 13, P = 0.037), and arterial elasticity (13 ± 5 vs. 21 ± 5, P = 0.012), as well as higher cardiac afterload (203 ± 57 vs. 151 ± 49, P = 0.015).
Conclusions: Using non-invasive ICG, we have shown that it is feasible to characterize hemodynamics in ambulatory HF patients. We show that AF compromises left ventricular function in patients with HF and is associated with excess afterload and reduced arterial elasticity.
Keywords: Atrial fibrillation; Heart failure; Hemodynamics; Impedance cardiography
| Introduction | ▴Top |
The deleterious effects of atrial fibrillation (AF) as part of heart failure (HF) are known to be associated with poor prognosis, which increases the mortality of HF by nearly 40% [1]. There are well known mechanisms through which AF impairs myocardial function including loss of cardiac output during tachycardia, the reduced ventricular filling due to the loss of atrial systole, and the activation of neurohormonal vasoconstrictor mechanisms [2, 3]. In fact, the mechanisms involved in hemodynamic alterations induced by AF are much more complex. Hemodynamic monitoring is frequently performed invasively in intensive care unit (ICU) among those who are hemodynamically unstable [4].
In patients with coexisting HF and AF, significant derangement of several hemodynamics parameters occurs, whether the heart rate during AF is controlled or not [5]. At the same time, performing invasive monitoring at these patients can be potentially harmful. Therefore a simplified comprehensive model to explain such hemodynamic processes could provide clinicians with better tools to assess, evaluate, and manage patients with coexisting HF and AF. Simple, reliable, and non-invasive tools to study these hemodynamic alterations in ambulatory patients could shed new light on the vicious electromechanical cycles observed in these patients.
In this pilot study, we sought to evaluate the clinical utility of impedance cardiography (ICG), a novel non-invasive tool for hemodynamic monitoring, in characterizing the electromechanical events seen in ambulatory, optimally managed HF patients. We also sought to compare the hemodynamics between those with and without AF.
| Materials and Methods | ▴Top |
Study population
This observational, correlational study enrolled ambulatory HF patients from an outpatient clinic in Newfoundland and Labrador, Canada. Inclusion criteria were the following: 1) over 18 years of age; and 2) New York Heart Association (NYHA) class I-III HF. We excluded patients with wide QRS duration (i.e., >120 ms) because of pacing or bundle branch block. The study was approved by the Health Research Ethics Authority in Newfoundland and Labrador in accordance with the ethical standards of the Helsinki Declaration. All participants signed the informed consent.
ICG
ICG is a non-invasive technique to continuously assess cardiac hemodynamics by measuring the dynamic changes in total electrical conductivity of the thorax at any given time [6]. ICG uses four pairs of sensing electrodes: two pairs are applied bilaterally to the base of the neck, and two pairs to the base of the thorax (Fig. 1). The transmitting electrodes send high frequency, low magnitude current through the thorax, and the sensing electrodes sense the changes in the impedance (i.e., resistance) of the flow of transmitted current. Given that electrical current seeks the path of least resistance, the transmitted current flows in the aorta during systole and the vena cava during diastole. Therefore, the dynamic changes in impedance from beat to beat correspond to the changes in blood volumes and velocity within the heart, which provides an opportunity for non-invasive quantification of many important hemodynamic parameters [7]. Table 1 summarizes seven clinically important hemodynamic parameters used in this study.
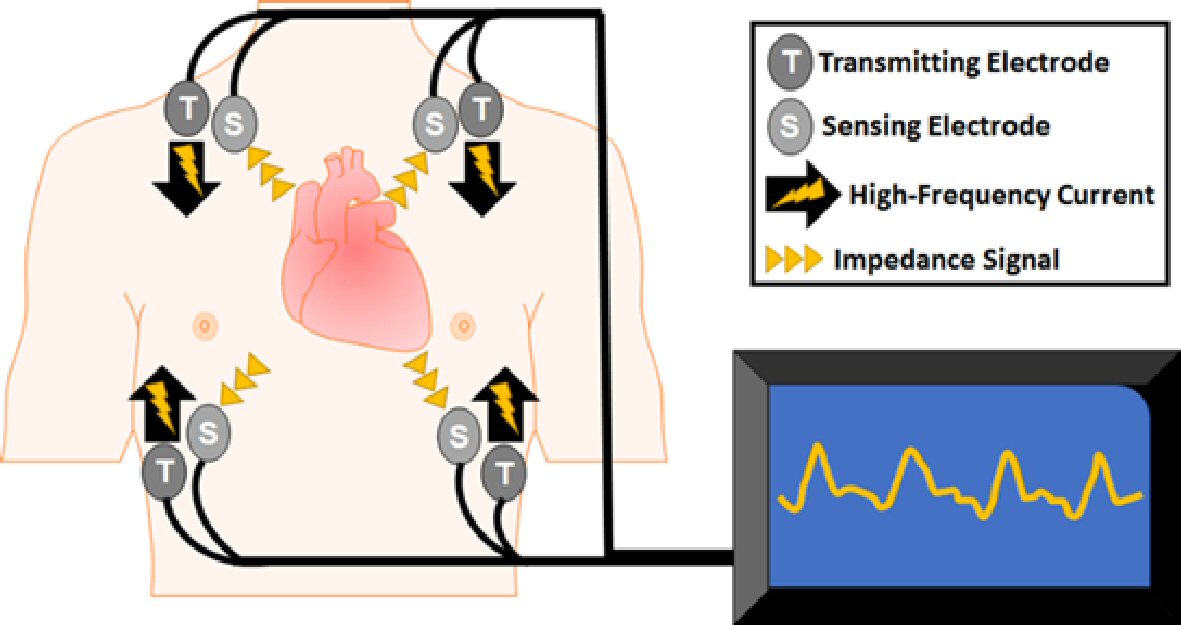 Click for large image | Figure 1. Impedance cardiography. Impedance cardiography is a non-invasive method to record changes in thoracic impedance using transmitting and sensing electrodes that are applied to the bases of the neck and thorax bilaterally. |
 Click to view | Table 1. Selected Hemodynamic Parameters Obtained by Impedance Cardiography (ICG) |
Data collection protocol
Ambulatory patients were invited to participate in our study during a routine outpatient clinic visit. After signing an informed consent form, participating patients were escorted to a private room where they donned a gown. The procedure of the study was explained and vital signs were obtained. The participants were then instructed to lie supine, while medical history and clinical data elements were obtained. Then, after preparation of a participant’s skin (i.e., removal of hair and sterilization with alcohol prep pads), wet gel ICG electrodes were applied to his or her chest. Electrocardiogram (ECG) and hemodynamic parameters of interest were analyzed with a BioZ Dx impedance cardiographer (Cardio Dynamics Inc., San Diego, CA, USA) and manufacturer specific algorithms. These parameters were then saved in an Excel spreadsheet for analysis. Participants were grouped according to the presence or absence of AF, with or without treatment, on their baseline ECG.
Statistical methods
All analyses were completed using IBM SPSS version 25, and the P value was set at 0.05 for two-tailed hypothesis testing. Values were reported as means plus or minus standard deviation (i.e., mean ± standard deviation (SD)) or as numbers and percentages (i.e., n and %). Patients with or without AF were compared using independent samples t-test for continuous variables, and Chi-square test for categorical variables. Correlations between continuous variables were evaluated using Pearson’s r correlation coefficient.
| Results | ▴Top |
The final sample of participants comprised 32 patients who were aged 62 ± 14 years. Table 1 summarizes the baseline characteristics of the study sample. The majority of participants were men (66%) who had NYHA HF class I (88%). The average ejection fraction (EF) observed was 0.33 ± 0.13, with nearly 70% of the participants exhibiting a reduced EF of less than 0.40. More than half of the participants had at least one cardiovascular comorbidity. All patients were on beta blockers and angiotensin-converting enzyme (ACE) inhibitors; 63% on diuretics; 30% on digoxin; and 13% on nitrates.
Overall, less than one-third of patients had AF (n = 9, 28%). Those with AF were heart rate controlled (80 ± 9) and their AF was chronic. There were no differences in baseline characteristics or home medications between those with or without AF. More importantly, there were significant differences between the groups in terms of cardiac hemodynamics (Fig. 2). Patients with AF exhibited lower stroke volume (SV) (60 ± 7 vs. 89 ± 29, P = 0.008), lower stroke work index (33 ± 9 vs. 45 ± 13, P = 0.016), lower velocity (30 ± 8 vs. 40 ± 13, P = 0.037), and lower total arterial compliance (13 ± 5 vs. 21 ± 5, P = 0.012), as well as higher systemic stroke resistance index (203 ± 57 vs. 151 ± 49, P = 0.015).
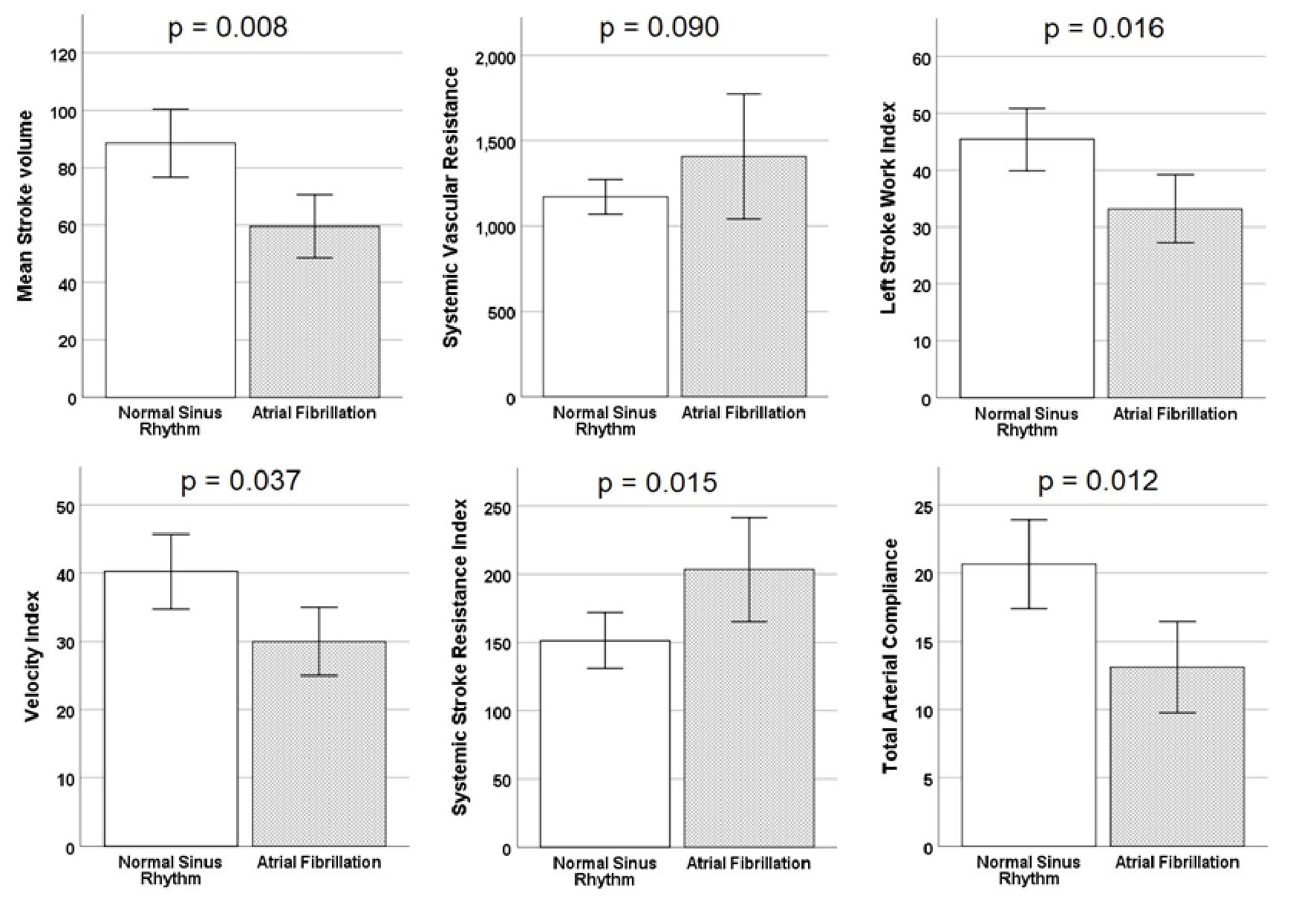 Click for large image | Figure 2. Association between hemodynamic parameters and heart rhythm. This figure shows the differences between the groups in terms of cardiac hemodynamics. Patients with atrial fibrillation exhibited lower stroke volume, lower stroke work index, lower velocity, and lower total arterial compliance, as well as higher systemic stroke resistance index. Error bars indicate ± SE. SE: standard error. |
Finally, we ran a multivariate logistic regression model using the ICG predictors as markers of AF and reported the resulting receiver operating characteristic (ROC) curve in Figure 3. The model demonstrates that these ICG parameters have high discriminant value for separating patients with and without AF with an area under ROC curve of 0.889.
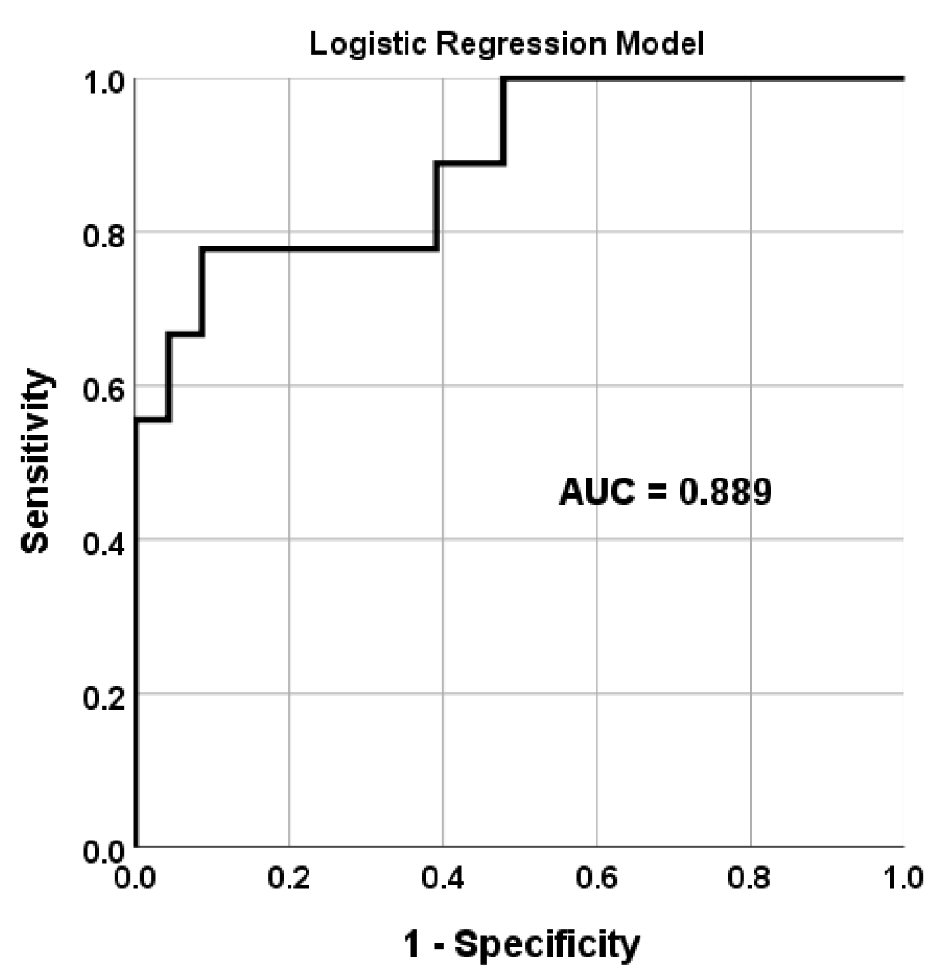 Click for large image | Figure 3. Discriminant value of ICG to identify patients with AF. This figure shows the discriminant value of ICG parameters reported in Table 1 for separating patients with and without AF. ROC corresponds to the predicted probability value of a multivariate logistic regression model. ICG: impedance cardiography; AF: atrial fibrillation; ROC: receiver operating characteristic; AUC: area under the ROC curve. |
| Discussion | ▴Top |
In this observational correlational study, we show that it is feasible to characterize hemodynamics in ambulatory HF patients using non-invasive ICG. Our results show that AF compromises left ventricular function (SV, workload, and contractility); and is associated with excess cardiac afterload and reduced arterial elasticity. These ICG parameters show excellent discriminant value to separate those with and without AF. Our findings are consistent with prior literature, suggesting that cardiac elasticity, resistance, and afterload can play a role in the onset AF and the progression of HF in these patients.
The cardiac function can be compromised in patients with coexisting HF and AF by number of ways: 1) AF negatively alters myocardial contractility by reducing SV and peak aortic blood flow, which both have a negative effect on cardiac function; 2) Increased systemic vascular resistance results in reduced arterial elasticity and increased beat-to-beat afterload, which both can lead to increased cardiac muscle strain and subsequent electromechanical derangement. The combination of inter-related measures of reduced arterial elasticity, increased afterload, and increased cardiac muscle strain seems to be upstream pathological processes that contribute to the initiation and progression of HF and AF, rather than being downstream consequences of the coexistence of HF and AF [8]. Nevertheless, vascular resistance and arterial elasticity are potential markers of endothelial dysfunction [9, 10]. Endothelial dysfunction is a plausible mechanistic link to explain the coexistence of HF and AF. We speculate that endothelial dysfunction, manifested in less vascular relaxation [11] which can result in excess afterload, atrial stretch, and left ventricular muscle strain [9, 12].
Effects on SV and EF
Although we found that participants with AF had lower SV compared to those without AF, both sets of participants (i.e., both with and without AF) exhibited an equivalent EF (Table 2). This suggests that patients with AF experience lower end-diastolic volume compared to their counterparts, which is expected because AF results in atrioventricular dyssynchrony, and a loss of atrial kick influence on preload. This deleterious effect can contribute to the activation of the renin-angiotensin-aldosterone system, which results in sustained increase in systemic vascular resistance [13].
 Click to view | Table 2. Baseline Sample Characteristics |
Effects on vascular resistance and arterial compliance
To maintain adequate end-organ perfusion in the presence of chronic HF, the sympathetic nervous system and neurohormonal systems (i.e., the renin-angiotensin system and antidiuretic hormone) remain activated. Jointly, these mechanisms accentuate systemic vasoconstriction and fluid overload, resulting in increased vascular resistance and arterial stiffness, loss of vascular relaxation, and cardiac remodeling [13].
These pathological conditions can contribute to the initiation and progression of HF. Nevertheless, our data demonstrate that AF is associated with more severe systemic vascular resistance and more diminished arterial compliance, which suggests that the initiation and progression of AF are associated with the kind of endothelial dysfunction frequently observed with acute HF [9].
Effects on afterload and left ventricle effort
Afterload is the pressure against which the heart must work to eject blood during systole. Afterload compromises two components: aortic pressure and cardiac wall tension. Our results demonstrate that AF is associated with higher systemic stroke resistance and subsequent lower peak aortic flow. Such elevated aortic pressure, which is influenced, in part, by the stiffness of aorta, results in increased left ventricular effort to overcome afterload. Nevertheless, our data also demonstrate that, despite the elevated systemic stroke resistance, patients with AF have a lower rather than higher left stroke work index, which reflects the deleterious effects of AF as part of HF.
Limitations
The study described in this paper has two major limitations. First, the sample size of participants recruited was small, which prevented more comprehensive sub-group analyses. However, our preliminary results establish the feasibility of subsequent work to study hemodynamics in ambulatory patients with HF. Second, no echocardiographic data on wall pressure or myocardial wall thickness was available. Such data could have provided insight into the pathological mechanisms involved in the observed relationships between AF, vascular resistance, and afterload. We encourage the inclusion of such data in subsequent studies.
Conclusions
This paper focuses on ICG as a simple and reliable tool for the non-invasive assessment of cardiac hemodynamics in ambulatory patients with HF. The study highlighted the associations between various hemodynamic variables among this patient population. We show that AF compromises left ventricular function in patients with HF, and is associated with excess afterload and reduced arterial elasticity. Our findings are consistent with prior literature and suggest that cardiac elasticity, resistance, and afterload can play a role in the onset of AF and the progression and worsening of HF in these patients. Yet, further research on these mechanisms in ambulatory patients might provide future opportunities for the development and testing of targeted treatment modalities.
Acknowledgments
None to declare.
Financial Disclosure
This study was funded by a grant from Memorial University of Newfoundland, Canada.
Conflict of Interest
None to declare.
Informed Consent
All participants signed the informed consent.
Authors Contribution
Mohammad Alhamaydeh: conceptualization, formal analysis, and writing (original draft); Fadi Khraim: project administration, supervision, funding acquisition, and writing (review and editing); Ziad Faramand: investigation and writing (review and editing); Samir Saba: supervision and writing (review and editing); Salah Al-Zaiti: conceptualization, methodology, formal analysis, writing (original draft), visualization, supervision. All authors have contributed to, read and approved the final manuscript for submission
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292.
- McMurray JJ, Petrie MC, Murdoch DR, Davie AP. Clinical epidemiology of heart failure: public and private health burden. Eur Heart J. 1998;19(Suppl P):P9-16.
- Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22(4 Suppl A):6A-13A.
doi - Redfield MM, Jacobsen SJ, Burnett JC, Jr., Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194-202.
doi pubmed - Meta-analysis Global Group in Chronic Heart Failure. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33(14):1750-1757.
doi pubmed - He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161(7):996-1002.
doi pubmed - Cha YM, Redfield MM, Shen WK, Gersh BJ. Atrial fibrillation and ventricular dysfunction: a vicious electromechanical cycle. Circulation. 2004;109(23):2839-2843.
doi pubmed - Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133(5):484-492.
doi pubmed - Mamas MA, Caldwell JC, Chacko S, Garratt CJ, Fath-Ordoubadi F, Neyses L. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail. 2009;11(7):676-683.
doi pubmed - Pozzoli M, Cioffi G, Traversi E, Pinna GD, Cobelli F, Tavazzi L. Predictors of primary atrial fibrillation and concomitant clinical and hemodynamic changes in patients with chronic heart failure: a prospective study in 344 patients with baseline sinus rhythm. J Am Coll Cardiol. 1998;32(1):197-204.
doi - Redfield MM, Kay GN, Jenkins LS, Mianulli M, Jensen DN, Ellenbogen KA. Tachycardia-related cardiomyopathy: a common cause of ventricular dysfunction in patients with atrial fibrillation referred for atrioventricular ablation. Mayo Clin Proc. 2000;75(8):790-795.
doi pubmed - Pinsky MR, Payen D. Functional hemodynamic monitoring. Crit Care. 2005;9(6):566-572.
doi pubmed - Bayram M, Yancy CW. Transthoracic impedance cardiography: a noninvasive method of hemodynamic assessment. Heart Fail Clin. 2009;5(2):161-168.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


