| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 12, Number 2, April 2021, pages 98-108
Effect of Ezetimibe Added to High-Intensity Statin Therapy on Low-Density Lipoprotein Cholesterol Levels: A Meta-Analysis
Jayden Leea, Ugochukwu Egolumb, Harish Pariharc, Michael Cooleya, Hua Linga, d
aSchool of Pharmacy, Philadelphia College of Osteopathic Medicine, Suwanee, GA, USA
bThe Heart Center of Northeast Georgia Medical Center, Gainesville, GA, USA
cCollege of Pharmacy, California Health Sciences University, Clovis, CA, USA
dCorresponding Author: Hua Ling, School of Pharmacy, Philadelphia College of Osteopathic Medicine, Georgia Campus, 625 Old Peachtree Road NW, Suwanee, GA 30024, USA
Manuscript submitted January 21, 2021, accepted January 28, 2021, published online February 23, 2021
Short title: Effect of Ezetimibe on LDL-C Levels
doi: https://doi.org/10.14740/cr1224
| Abstract | ▴Top |
Background: Adding ezetimibe to high-intensity statin therapy is used for additional lowering of low-density lipoprotein cholesterol (LDL-C); however, there are little data on the efficacy of ezetimibe when combined with a high-intensity statin. A meta-analysis was performed to evaluate the efficacy of ezetimibe added to high-intensity statin therapy on LDL-C levels.
Methods: A literature search from database inception to May 2020 was performed using PubMed, EMBASE and Cochrane Central Register of Controlled Trials. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were used in this meta-analysis, in which the random-effects model was adopted for the calculation of the mean difference (MD). The Cochrane Collaboration’s tool for assessing the risk of bias was used to evaluate the quality of the included trials.
Results: A total of 14 trials with 2,007 patients were included in this study. Compared to the high-intensity statin monotherapy, the MD in LDL-C reduction with high-intensity statin therapy plus ezetimibe was -14.00% (95% confidence interval: -17.78 to -10.22; P < 0.001) with a moderate degree of heterogeneity (P < 0.001, I2 = 66%). No significant publication bias among the included trials was identified.
Conclusions: Our study found that adding ezetimibe to high-intensity statin therapy provided a significant but attenuated incremental reduction in LDL-C levels. Whether the magnitude of this additional lowering of LDL-C levels would lead to benefits in clinical cardiovascular outcomes needs further investigation.
Keywords: Ezetimibe; High-intensity statin; LDL-C; Hypercholesterolemia; Coronary heart disease
| Introduction | ▴Top |
Ezetimibe is the most used non-statin medication for secondary prevention in patients with clinical atherosclerotic cardiovascular disease (ASCVD). It was recommended by the 2018 AHA/ACC Guideline on the Management of Blood Cholesterol [1] (2018 AHA/ACC Cholesterol Guideline) for patients with clinical ASCVD receiving maximally tolerated statin therapy when the low-density lipoprotein cholesterol (LDL-C) level remains ≥ 1.8 mmol/L (70 mg/dL). Ezetimibe was also recommended in patients with severe hypercholesterolemia on maximally tolerated statin therapy and the LDL-C level ≥ 2.6 mmol/L (100 mg/dL).
However, there is a lack of high-quality evidence for these moderate or weak recommendations, with no level A evidence provided in the 2018 AHA/ACC Cholesterol Guideline. In real-world practice, the maximally tolerated statin therapy is a high-intensity statin for majority patients, as evidenced by a prospective cohort study showing that approximately 70% of patients took a high-intensity statin at 1 year after acute coronary syndromes (ACS) [2]. Moreover, 12.6% of patients with ACS in the study were on the combination therapy of a statin and ezetimibe, and among them, about 60% of patients took a high-intensity statin and ezetimibe [2]. Unfortunately, there have been no large-scale randomized double-blinded clinical trials to evaluate the combination of high-intensity statin therapy and ezetimibe. The only available outcome study that was cited in the guidelines was the IMPROVE-IT trial [3], in which a moderate-intensive statin was used and ezetimibe only demonstrated a modest reduction in cardiovascular events. Whether the modest benefit observed in the IMPROVE-IT trial can be extrapolated into the real-world patients taking ezetimibe with a high-intensity statin is unknown. The possibility that the co-administration of high-intensity statin may offset the limited benefits of ezetimibe, making the addition of ezetimibe unnecessary cannot be ruled out.
As outcome trial evaluating the combination of high-intensity statin and ezetimibe was not available, the LDL-C levels as a surrogate endpoint could be used alternatively to evaluate the benefits of ezetimibe when adding to the high-intensity statin therapy. Several small trials on this topic have been conducted, but results are conflicting. The 2018 AHA/ACC Cholesterol Guideline stated that “The addition of ezetimibe or bile acid sequestrants to statin therapy typically provides an additional 15% to 25% reduction in LDL-C” [1], but, oddly, no reference regarding this statement was provided in the guideline. Thus, there is a gap of evidence in the medical literature to support this statement.
Numerous meta-analysis studies investigating the efficacy of ezetimibe added to ongoing statin therapy on LDL-C levels were reported, but none of them has evaluated the addition of ezetimibe to high-intensity statin therapy. Mikhailidis et al [4] conducted a meta-analysis including five randomized controlled trials involving a total of 5,039 patients; however, only low- to moderate-intensity statins were used in these five trials. Four years later, Mikhailidis et al [5] reported another meta-analysis about ezetimibe of 13 trials including 5,080 patients, but only one included trial employed high-intensity statin. Savarese et al [6] and Ye et al [7] also evaluated the combination of ezetimibe and statin therapy, respectively, but neither of them included trials adding ezetimibe to high-intensity statin therapy. Recently, Yu et al [8] conducted a meta-analysis comparing the efficacy of combination therapy with ezetimibe and statins versus a double dose of statin monotherapy. Among the 11 included studies, only one included trial had a therapy group receiving the combination of ezetimibe and a high-intensity statin. The most comprehensive meta-analysis of ezetimibe was reported by Lorenzi et al [9], and a total of 35 randomized controlled trials were identified and included in their analysis. Of them, only two trials had intervention groups receiving the combination of high-intensity statin therapy and ezetimibe. Besides, ezetimibe has been used as a positive control in several clinical trials [10-13] of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and cholesteryl ester transfer protein (CETP) inhibitors; however, those trials have not been evaluated in the previous meta-analysis studies.
To the best of our knowledge, there is neither meta-analysis nor large-scale outcome trial examining the addition of ezetimibe to the background high-intensity statin therapy reported yet. Here, we performed a meta-analysis to add some insights into this topic by evaluating the efficacy of ezetimibe in reducing LDL-C levels when added to high-intensity statin therapy, and a discussion was also presented regarding the relationship between the magnitude of LDL-C lowering of ezetimibe and the reduction of ASCVD risk.
| Materials and Methods | ▴Top |
Literature search
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting the present meta-analysis. A systematic literature search was performed through May 2020 using PubMed (1946-), EMBASE (1947-) and Cochrane Central Register of Controlled Trials with the following key terms: “atorvastatin”, “rosuvastatin”, “high”, “statin” and “ezetimibe” (Supplementary Material 1, www.cardiologyres.org). The screening was restricted to clinical trials in humans published in English. The references were imported into EndNote™ and then Rayyan, which is a systematic reviews web app. Duplicate records were removed automatically.
Article screening and selection
Two authors independently assessed the search result for eligibility by title and/or abstract using Rayyan. Differences were resolved through consensus between the authors. Studies that met the following criteria were included in the meta-analysis: 1) Comparing high-intensity statin plus ezetimibe with the corresponding high-intensity statin monotherapy in patients with a history of clinical ASCVD or hypercholesterolemia; 2) Having comparable baseline LDL-C levels in both groups; 3) Reporting the change in LDL-C levels. Studies containing only a single group (i.e., pre-post treatment) were excluded because they did not contain an adequate comparison group. High-intensity statin therapy was defined as atorvastatin 40 - 80 mg daily or rosuvastatin 20 - 40 mg daily [14]. References passed the initial screening were further evaluated through the full-text review.
Data extraction and statistical analysis
The study design and demographic characteristics of the selected trials, including trial settings, trial locations, sample size, study population, interventions and the changes of LDL-C levels were extracted and assessed. The Cochrane Collaboration’s tool for assessing the risk of bias was used, and two authors independently evaluated the quality of the included trials. Like the article screening process, differences were resolved through consensus between the authors.
The outcome measure of this analysis was the mean difference (MD) in the reduction of LDL-C levels in patients treated with the combination of a high-intensity statin and ezetimibe vs. the corresponding high-intensity statin monotherapy. The random-effects model with the DerSimonian-Laird method was adopted for the calculation of MD given expected clinical and methodological heterogeneity. Three subgroup analyses were conducted based on the type of high-intensity statins (atorvastatin vs. rosuvastatin), the dose of high-intensity statins (low dose (atorvastatin 40 mg and rosuvastatin 20 mg) vs. high dose (atorvastatin 80 mg and rosuvastatin 40 mg)) and study regions (Asia vs. Western countries). A meta-regression analysis using the random-effects model (method of moments) was performed to evaluate the effect of baseline LDL-C levels. The I2 statistic test for assessment of in-between study heterogeneity with values < 25%, 25-50%, 50-75% and > 75% corresponds to no, low, moderate and high degree of heterogeneity, respectively. Small study effects were evaluated through Egger’s test and Begg’s test. We used a confidence interval (CI) of 95% and P < 0.05 as a reflection of statistically significant results in all our analyses. All statistical analyses were conducted by RevMan 5.3 and jamovi 1.1.9.
| Results | ▴Top |
Literature search and study characteristics
Of the initial 1,037 citations, 14 clinical trials involving 2,007 patients were included in this analysis (Fig. 1) [10, 12, 13, 15-25]. Among them, the study of Nicholls 2017 did not report standard deviations (SDs), and the missing SDs were borrowed from the study of Robinson 2014 due to similarity between these two trials according to the recommendations of Cochrane Handbook for Systematic Reviews of Interventions. The study of Ballantyne 2003 contains two treatment arms of high-intensity statins (atorvastatin 40 mg and 80 mg), and the results of these two arms were reported separately. Thus, in our meta-analysis, the results of these two arms were separately analyzed accordingly though they were from the same study.
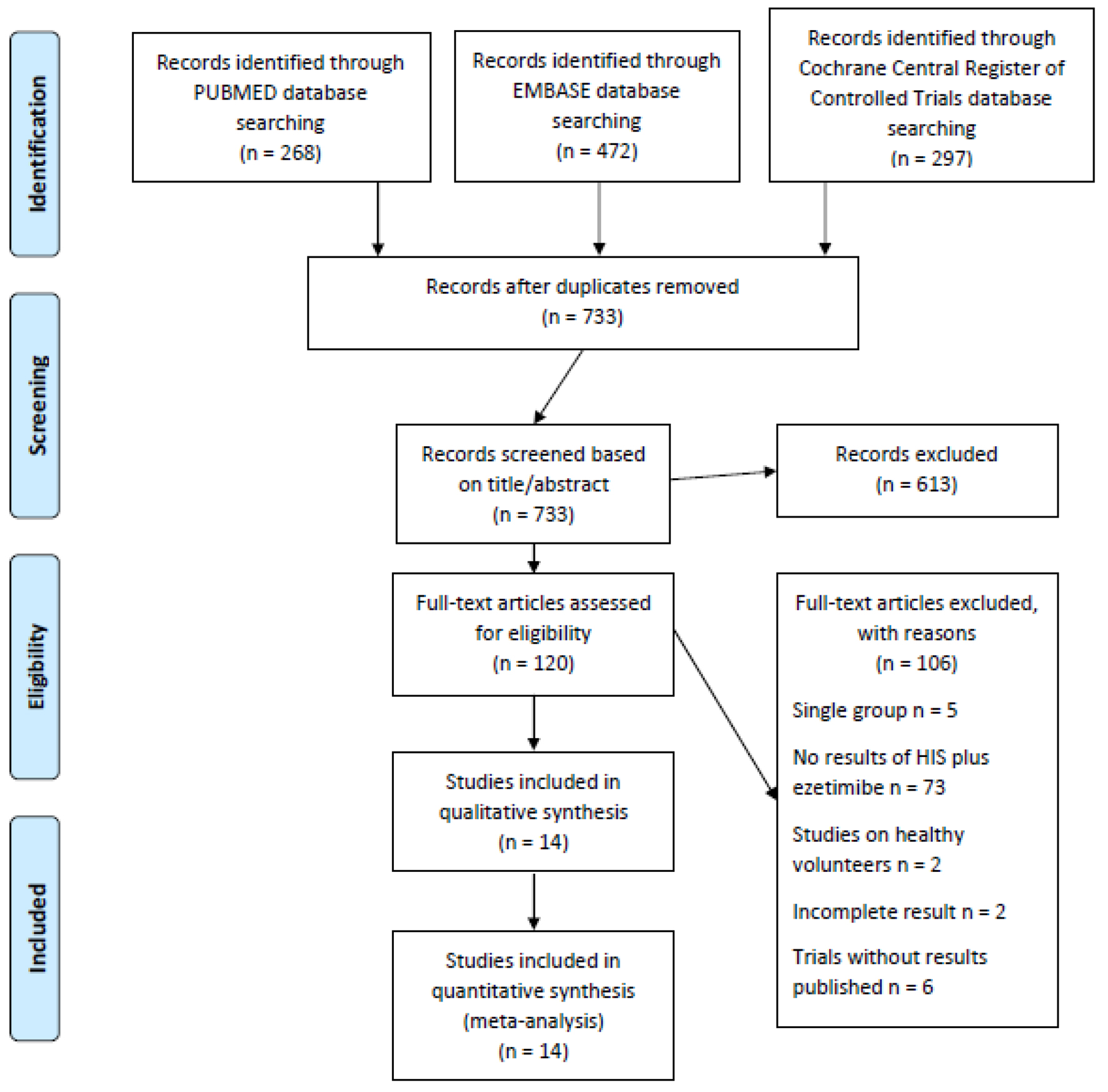 Click for large image | Figure 1. Flowchart for study selection. |
The characteristics of the included trials were summarized in Table 1 [10, 12, 13, 15-25]. Four included studies were open-label trials, and two double-blinded studies did not mask ezetimibe use. Three trials were designed to evaluate PCSK9 inhibitors or CETP inhibitors, and the data about ezetimibe were a by-product [9, 11, 12]. A mean LDL-C level of < 1.8 mmol/L (70 mg/dL) was achieved more frequently in the groups of patients receiving the ezetimibe plus high-intensity statin therapy than the groups of patients receiving high-intensity statin monotherapy in the included trials. Of note, in the study of Robinson 2014, a more than 10% increase in the LDL-C levels from baseline in the atorvastatin 80 mg monotherapy group was reported. It is unclear why the LDL-C levels were elevated despite the continuous atorvastatin 80 mg monotherapy after a 4-week lipid stabilization period.
 Click to view | Table 1. Study Characteristics |
The overall risk of bias was low or unclear, except for four open-label trials with a high risk of performance bias due to the nature of their study design (Fig. 2). Visual inspection of the funnel plot (Supplementary Material 2, www.cardiologyres.org) suggests some extent of asymmetry, but no statistically significant publication bias among the included trials was identified (Egger’s test, P = 0.702; Begg’s test, P = 0.282).
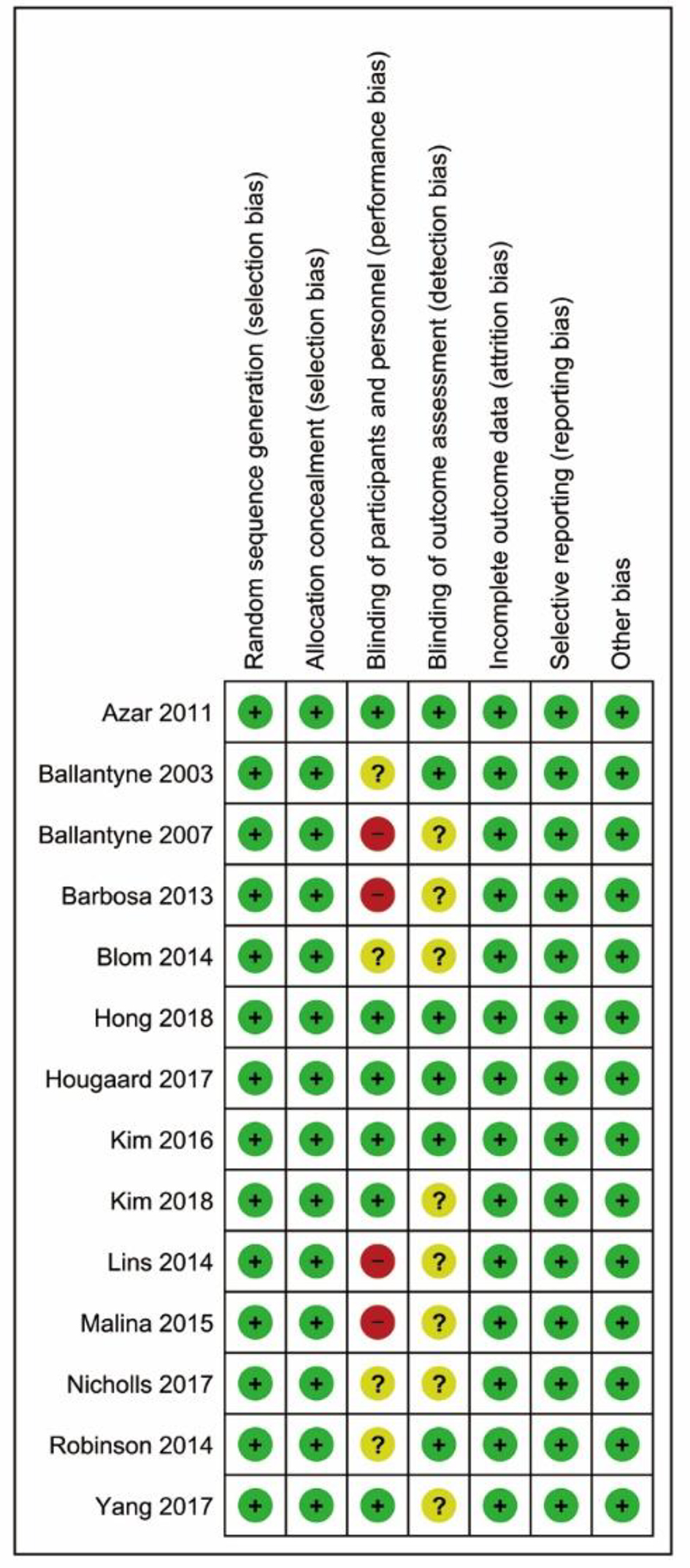 Click for large image | Figure 2. Risk of bias summary. |
The MD in LDL-C levels and sensitivity analyses
Compared to the high-intensity statin monotherapy, the MD in LDL-C levels with a high-intensity statin plus ezetimibe was -14.00% (95% CI: -17.78 to -10.22; P < 0.001) (Fig. 3). The result was associated with a moderate degree of heterogeneity (I2 = 66%, P < 0.001). The exclusion sensitivity analysis showed that the heterogeneity was mainly contributed by the study of Robinson 2014 (Table 2). When this study was omitted, the MD in LDL-C levels was changed to -12.09% (95% CI: -14.64 to -9.55; P < 0.001) with insignificant heterogeneity (P = 0.226, I2 = 21%).
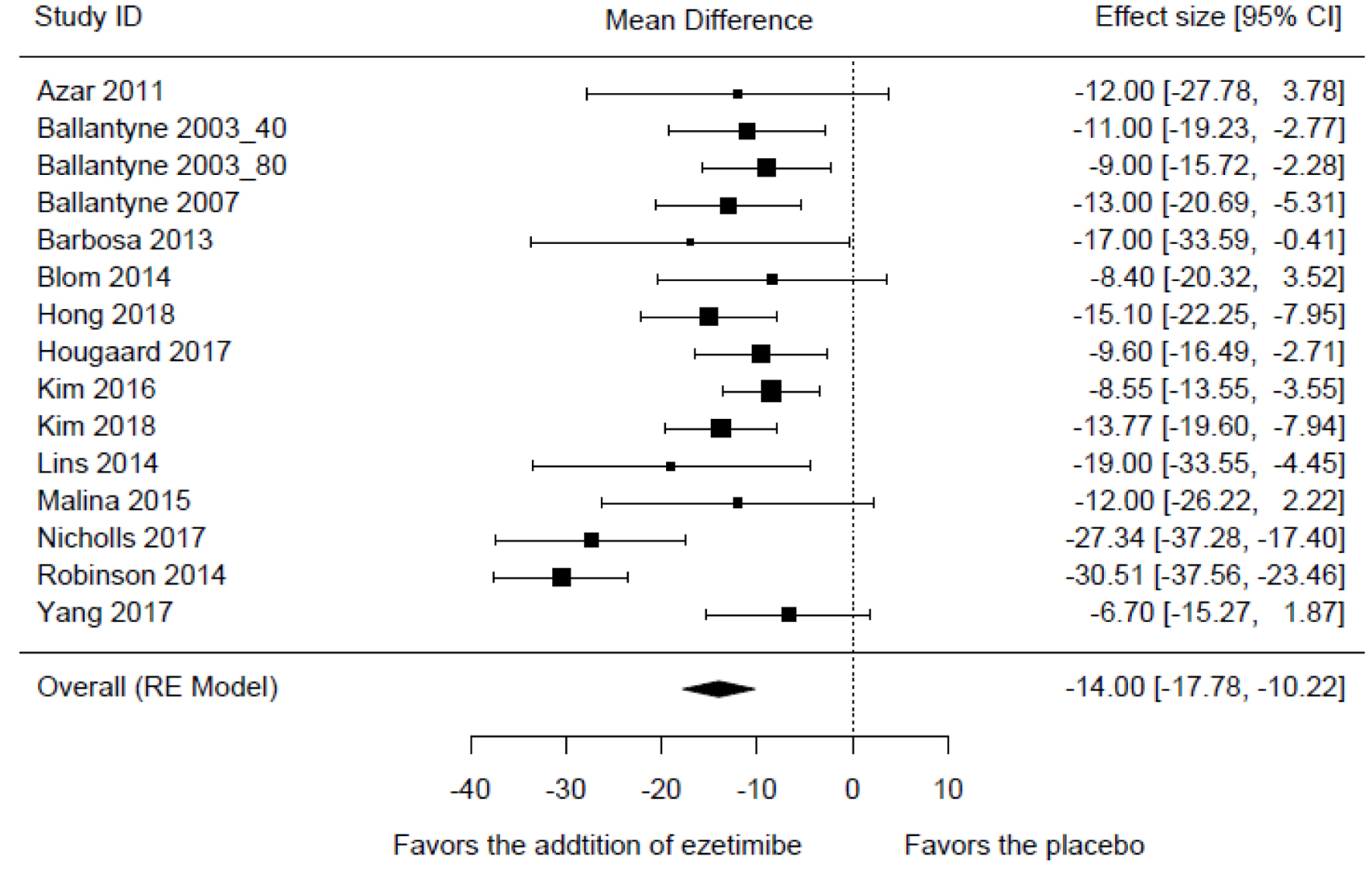 Click for large image | Figure 3. Forest plot of comparison: the mean difference in the reduction of LDL-C levels. LDL-C: low-density lipoprotein cholesterol. |
 Click to view | Table 2. Exclusion Sensitivity Analysis |
Subgroup analysis and meta-regression
Subgroup analysis yielded non-significant differences between the types of high-intensity statins (P = 0.204), the doses of high-intensity statins (P = 0.838) and the trial regions (P = 0.109). The meta-regression analysis demonstrated a significant effect of baseline LDL-C levels with a coefficient of 0.123 (standard error = 0.044; P = 0.006). However, it should be noted that the significance was eliminated after removing the study of Robinson 2014 (P = 0.055).
| Discussion | ▴Top |
Here we reported a meta-analysis of 14 clinical trials with 2,007 patients included. The study found that adding ezetimibe to high-intensity statin led to a 14% incremental lowering in LDL-C levels compared to high-intensity statin monotherapy, which was statistically significant. This additional lowering of LDL-C levels was irrespective of the types of high-intensity statin or the trial regions. Also doubling the dose of high-intensity statins did not influence the effect of ezetimibe significantly. Our findings support the statement about the efficacy of ezetimibe in reducing LDL-C levels in 2018 AHA/ACC Cholesterol Guideline.
The trend of our findings was consistent with the results of the IMPROVE-IT trial. In the IMPROVE-IT trial [3], ezetimibe 10 mg daily additionally decreased LDL-C levels by 24% (0.43 mmol/L (16.7 mg/dL), P < 0.001) compared to placebo when added to background therapy of simvastatin 40 mg daily. Unlike the IMPROVE-IT trial, our meta-analysis identified a less percentage change in LDL-C levels, indicating that the impact of ezetimibe on reducing LDL-C levels may be lessened by co-administration with a high-intensity statin.
A similar pattern was observed in the ENHANCE trial as well. In the ENHANCE trial [26], ezetimibe added to background therapy of simvastatin 80 mg daily demonstrated an additional reduction in LDL-C levels by 16.5% (P < 0.01) compared to placebo in patients with familial hypercholesterolemia. The potency of simvastatin 80 mg was slightly less than atorvastatin 40 mg with a roughly 48% LDL-C reduction. The ENHANCE trial resulted in a less percentage change in LDL-C levels compared to the IMPROVE-IT trial, but slightly more percentage change in LDL-C levels compared to our meta-analysis. The differences between these three studies may also suggest that the impact of ezetimibe on reducing LDL-C levels depends on the intensity of background statin therapy.
In the previous meta-analysis studies, considerably more reduction of LDL-C levels was observed when adding ezetimibe to low- to moderate-intensity statins. In the study of Mikhailidis et al [4], a -23.6% reduction of LDL-C levels was reported when adding ezetimibe to low- to moderate-intensity statin (simvastatin 10 - 20 mg and atorvastatin 10 - 20 mg). In Mikhailidis et al’s later study [5] comparing the efficacy of the addition of ezetimibe to statin vs. doubling statin, the addition of ezetimibe to statin therapy resulted in a roughly -20% greater reduction of LDL-C levels after adjusted the effect of doubling statin dose with the 6% rule. All the included trials used low to moderate statins (atorvastatin 10 - 20 mg and simvastatin 10 - 40 mg), except one trial with atorvastatin 40 mg. In the most comprehensive meta-analysis involving 35 trials [9], the addition of ezetimibe led to an MD in LDL-C of -13.62%, -14.71% and -14.96%, compared to doubling the starting dose of simvastatin, atorvastatin and rosuvastatin, respectively. The results were consistent with Mikhailidis et al’s study, and an approximately -20% additional lowering of LDL-C was achieved if the effect of doubling dose was adjusted with the 6% rule. Notably, among the 35 included trials, only two trials used high-intensity statin as the background therapy (atorvastatin 40 mg [27]; atorvastatin 10 mg or 20 mg or 40 mg or 80 mg [28]). In a pooled analysis of over 21,000 subjects from 27 clinical trials [29], a significant incremental reduction of -23.4% in LDL-C levels was attributable to the addition of ezetimibe compared to the control groups. Similar to the meta-analysis studies, the low- to moderate-intensity statin was found in the majority of the included trials in this pooled analysis. Overall, adding ezetimibe to low- to moderate-intensity statin was associated with an approximately 20-24% extra reduction of LDL-C levels, which was greater than the MD reported in our meta-analysis of adding ezetimibe to high-intensity statin therapy. Taking into account the results of our meta-analysis, the variation in the efficacy of ezetimibe on LDL-C levels could be explained by the different strengths of the background statin therapy.
Though more patients achieved a target LDL-C level of < 1.8 mmol/L (70 mg/dL) when adding ezetimibe to the high-intensity statin therapy in the included trials, it was well established that the ASCVD risk reduction was associated with the magnitude of the lowering of LDL-C levels [30-32]. As described in the IMPROVE-IT trial, adding ezetimibe to a moderate-intensive statin was associated with a 6.4% relative risk reduction in cardiovascular outcomes (absolute risk reduction, 2.0%; hazard ratio, 0.936; 95% CI, 0.89 - 0.99; P = 0.016). While other factors also play an important role in influencing the cardiovascular outcome, the percentage reduction in LDL-C levels from baseline was linearly correlated with ASCVD rates [31]. Hence, it was very unlikely that the proportional benefit of ezetimibe in reducing major vascular events, translated from the 14% reduction in LDL-C levels, could be the same as the modest benefit reported in the IMPROVE-IT trial. We hypothesized that the magnitude of the clinical benefit of adding ezetimibe to a high-intensity statin in reducing cardiovascular events might not be clinically significant. Unfortunately, at the time of writing, there is no good evidence for the effectiveness of ezetimibe in reducing cardiovascular events with the background high-intensity statin therapy. Without any patient-level data, the clinical significance of adding ezetimibe to high-intensity statin therapy in reducing cardiovascular events remains unclear. Though ezetimibe is well tolerated when combined with statin therapy, the cardiovascular benefits of adding ezetimibe to the background high-intensity statin therapy beyond lipids lowering have to be examined to support the use of ezetimibe.
Limitations
Our study has several limitations. First, this meta-analysis is limited by the small number of included trials, the small sample size of each trial and the various trial settings, as shown in Table 1. Second, some of the included trials about PCSK9 inhibitors or CETP inhibitors were not designed to evaluate the effectiveness of ezetimibe on LDL-C levels. Third, the use of statins or ezetimibe was not masked in two trials in addition to four open-label trials, potentially increased risk of performance risk. Fourth, the moderate degree of heterogeneity caused by the study of Robinson 2014 might be explained by the small-study effects, as there were only about 100 patients in each group. Without any additional information or explanation provided by the article, we were unable to find a conclusive reason of the heterogeneity. Thus, we accept the existence of the heterogeneity and the potential greater uncertainty within our results. Taken together, our results should be interpreted with caution.
Conclusions
In summary, adding ezetimibe to high-intensity statin therapy provided a significant but attenuated additional reduction in LDL-C levels. However, whether this further lowering of LDL-C levels achieved by adding ezetimibe to a high-intensity statin would lead to a benefit in cardiovascular outcomes needs further investigation. Our study bridges the gap in the medical literature of the clinic role of ezetimibe with high-intensity statin therapy, and reinforces the statement in the 2018 AHA/ACC Cholesterol Guideline about the efficacy of ezetimibe in reducing LDL-C levels.
| Supplementary Material | ▴Top |
Suppl 1. Detailed Search Strategy Used in Each Database.
Suppl 2. Funnel plot of the 14 studies included in the meta-analysis.
Acknowledgments
None to declare.
Financial Disclosure
Dr. Egolum consults/advisory board for Akcea Therapeutics, Alnylam, AstraZeneca and Pfizer. Dr. Ling serves on an advisory board for Alnylam. The remaining authors have nothing to disclose.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
HL contributed to the conception and design of the work. JL, HP and MC contributed to the data acquisition, analysis, and interpretation of data for the work. JL and HL drafted the manuscript. UE critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139(25):e1082-e1143.
doi - Gencer B, Carballo D, Nanchen D, Koskinas KC, Klingenberg R, Raber L, Auer R, et al. Intensified lipid lowering using ezetimibe after publication of the IMPROVE-IT trial: A contemporary analysis from the SPUM-ACS cohort. Int J Cardiol. 2020;303:8-13.
doi pubmed - Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015;372(25):2387-2397.
doi pubmed - Mikhailidis DP, Sibbring GC, Ballantyne CM, Davies GM, Catapano AL. Meta-analysis of the cholesterol-lowering effect of ezetimibe added to ongoing statin therapy. Curr Med Res Opin. 2007;23(8):2009-2026.
doi pubmed - Mikhailidis DP, Lawson RW, McCormick AL, Sibbring GC, Tershakovec AM, Davies GM, Tunceli K. Comparative efficacy of the addition of ezetimibe to statin vs statin titration in patients with hypercholesterolaemia: systematic review and meta-analysis. Curr Med Res Opin. 2011;27(6):1191-1210.
doi pubmed - Savarese G, De Ferrari GM, Rosano GM, Perrone-Filardi P. Safety and efficacy of ezetimibe: A meta-analysis. Int J Cardiol. 2015;201:247-252.
doi pubmed - Ye Y, Zhao X, Zhai G, Guo L, Tian Z, Zhang S. Effect of high-dose statin versus low-dose statin plus ezetimibe on endothelial function: a meta-analysis of randomized trials. J Cardiovasc Pharmacol Ther. 2012;17(4):357-365.
doi pubmed - Yu M, Liang C, Kong Q, Wang Y, Li M. Efficacy of combination therapy with ezetimibe and statins versus a double dose of statin monotherapy in participants with hypercholesterolemia: a meta-analysis of literature. Lipids Health Dis. 2020;19(1):1.
doi pubmed - Lorenzi M, Ambegaonkar B, Baxter CA, Jansen J, Zoratti MJ, Davies G. Ezetimibe in high-risk, previously treated statin patients: a systematic review and network meta-analysis of lipid efficacy. Clin Res Cardiol. 2019;108(5):487-509.
doi pubmed - Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, Ceska R, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370(19):1809-1819.
doi pubmed - Farnier M, Jones P, Severance R, Averna M, Steinhagen-Thiessen E, Colhoun HM, Du Y, et al. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular-risk patients: The ODYSSEY OPTIONS II randomized trial. Atherosclerosis. 2016;244:138-146.
doi pubmed - Nicholls SJ, Ray KK, Ballantyne CM, Beacham LA, Miller DL, Ruotolo G, Nissen SE, et al. Comparative effects of cholesteryl ester transfer protein inhibition, statin or ezetimibe on lipid factors: The ACCENTUATE trial. Atherosclerosis. 2017;261:12-18.
doi pubmed - Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, Somaratne R, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA. 2014;311(18):1870-1882.
doi pubmed - Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139(25):e1046-e1081.
doi - Azar M, Valentin E, Badaoui G, Kassab R, Sarkis A, Azar RR. Comparison of the effects of combination atorvastatin (40 mg) + ezetimibe (10 mg) versus atorvastatin (40 mg) alone on secretory phospholipase A2 activity in patients with stable coronary artery disease or coronary artery disease equivalent. Am J Cardiol. 2011;107(11):1571-1574.
doi pubmed - Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, Sun S, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107(19):2409-2415.
doi pubmed - Ballantyne CM, Weiss R, Moccetti T, Vogt A, Eber B, Sosef F, Duffield E, et al. Efficacy and safety of rosuvastatin 40 mg alone or in combination with ezetimibe in patients at high risk of cardiovascular disease (results from the EXPLORER study). Am J Cardiol. 2007;99(5):673-680.
doi pubmed - Barbosa SP, Lins LC, Fonseca FA, Matos LN, Aguirre AC, Bianco HT, Amaral JB, et al. Effects of ezetimibe on markers of synthesis and absorption of cholesterol in high-risk patients with elevated C-reactive protein. Life Sci. 2013;92(14-16):845-851.
doi pubmed - Hong SJ, Jeong HS, Ahn JC, Cha DH, Won KH, Kim W, Cho SK, et al. A phase III, multicenter, randomized, double-blind, active comparator clinical trial to compare the efficacy and safety of combination therapy with ezetimibe and rosuvastatin versus rosuvastatin monotherapy in patients with hypercholesterolemia: I-ROSETTE (Ildong Rosuvastatin & Ezetimibe for Hypercholesterolemia) randomized controlled trial. Clin Ther. 2018;40(2):226-241 e224.
doi pubmed - Hougaard M, Hansen HS, Thayssen P, Antonsen L, Junker A, Veien K, Jensen LO. Influence of ezetimibe in addition to high-dose atorvastatin therapy on plaque composition in patients with ST-segment elevation myocardial infarction assessed by serial: Intravascular ultrasound with iMap: the OCTIVUS trial. Cardiovasc Revasc Med. 2017;18(2):110-117.
doi pubmed - Kim KJ, Kim SH, Yoon YW, Rha SW, Hong SJ, Kwak CH, Kim W, et al. Effect of fixed-dose combinations of ezetimibe plus rosuvastatin in patients with primary hypercholesterolemia: MRS-ROZE (Multicenter Randomized Study of ROsuvastatin and eZEtimibe). Cardiovasc Ther. 2016;34(5):371-382.
doi pubmed - Kim W, Yoon YE, Shin SH, Bae JW, Hong BK, Hong SJ, Sung KC, et al. Efficacy and safety of ezetimibe and rosuvastatin combination therapy versus those of rosuvastatin monotherapy in patients with primary hypercholesterolemia. Clin Ther. 2018;40(6):993-1013.
doi pubmed - Lins LC, Franca CN, Fonseca FA, Barbosa SP, Matos LN, Aguirre AC, Bianco HT, et al. Effects of ezetimibe on endothelial progenitor cells and microparticles in high-risk patients. Cell Biochem Biophys. 2014;70(1):687-696.
doi pubmed - Malina DM, Fonseca FA, Barbosa SA, Kasmas SH, Machado VA, Franca CN, Borges NC, et al. Additive effects of plant sterols supplementation in addition to different lipid-lowering regimens. J Clin Lipidol. 2015;9(4):542-552.
doi pubmed - Yang YJ, Lee SH, Kim BS, Cho YK, Cho HJ, Cho KI, Kim SY, et al. Combination Therapy of Rosuvastatin and Ezetimibe in Patients with High Cardiovascular Risk. Clin Ther. 2017;39(1):107-117.
doi pubmed - Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, Visseren FL, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358(14):1431-1443.
doi pubmed - Leiter LA, Bays H, Conard S, Bird S, Rubino J, Hanson ME, Tomassini JE, et al. Efficacy and safety of ezetimibe added on to atorvastatin (40 mg) compared with uptitration of atorvastatin (to 80 mg) in hypercholesterolemic patients at high risk of coronary heart disease. Am J Cardiol. 2008;102(11):1495-1501.
doi pubmed - Pearson TA, Denke MA, McBride PE, Battisti WP, Brady WE, Palmisano J. A community-based, randomized trial of ezetimibe added to statin therapy to attain NCEP ATP III goals for LDL cholesterol in hypercholesterolemic patients: the ezetimibe add-on to statin for effectiveness (EASE) trial. Mayo Clin Proc. 2005;80(5):587-595.
doi pubmed - Morrone D, Weintraub WS, Toth PP, Hanson ME, Lowe RS, Lin J, Shah AK, et al. Lipid-altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: a pooled analysis of over 21,000 subjects from 27 clinical trials. Atherosclerosis. 2012;223(2):251-261.
doi pubmed - Cholesterol Treatment Trialists Collaboration, Fulcher J, O'Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385(9976):1397-1405.
doi - Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316(12):1289-1297.
doi pubmed - Navarese EP, Robinson JG, Kowalewski M, Kolodziejczak M, Andreotti F, Bliden K, Tantry U, et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA. 2018;319(15):1566-1579.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


