| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 13, Number 6, December 2022, pages 315-322
Impact of Fried Frailty Phenotype on Postoperative Outcomes After Durable Contemporary Mechanical Circulatory Support: A Single-Center Experience
Temitope Ajibawoa, c , Priyank Chauhana, Radha Gopalanb
aDepartment of Medicine, Banner University Medical Center, Phoenix, AZ 85006, USA
bDivision of Cardiology, Department of Medicine, Banner University Medical Center, Phoenix, AZ 85006, USA
cCorresponding Author: Temitope Ajibawo, Department of Medicine, Banner University Medical Center, Phoenix, AZ 85006, USA
Manuscript submitted August 22, 2022, accepted October 8, 2022, published online December 16, 2022
Short title: Fried Frailty Phenotype in MCS Devices
doi: https://doi.org/10.14740/cr1423
| Abstract | ▴Top |
Background: Frailty is prevalent in advanced heart failure patients and may help distinguish patients at risk of worse outcomes. However, the effect of frailty on postoperative clinical outcomes is still understudied. Therefore, we aim to study the relationship between frailty and postoperative clinical outcomes in patients undergoing long-term mechanical circulatory support (MCS).
Methods: Forty-six patients undergoing durable MCS (left ventricular assist device and total artificial heart) placement at our medical center were assessed for frailty pre-implant. Frailty was defined as ≥ 3 physical components of the Fried frailty phenotype. Our primary endpoint is 1 year of survival post-implant. Secondary endpoints include 30-day all-cause rehospitalization, pump thrombosis, neurological event (stroke/transient ischemic attack), gastrointestinal bleeding, and driveline infection within 12 months post-MCS support.
Results: Of the 46 patients, 32 (69%) met the criteria for frailty according to Fried. The cohort’s median age was 67.0 years. The frail group had statistically significant lower left ventricular ejection fraction (LVEF) (11% vs. 20%, P = 0.017) and lower albumin (3.5 vs. 4.0 g/dL, P = 0.021). The frail cohort also had significantly higher rates of comorbid chronic kidney disease (47% vs. 7%, P = 0.016). There were no differences between the frail vs. non-frail group in terms of 30-day readmission rates (40% vs. 39%, P = 0.927) and 1-year post-intervention survival (log-rank, P = 0.165). None of the other secondary endpoints reached statistical significance, although the incidence of gastrointestinal bleed (24% vs. 16%, P = 0.689) and pump thrombosis (8% vs. 0%, P = 0.538) were higher in the frail group.
Conclusions: Preoperative Fried frailty was not associated with readmission at 30 days, mortality at 365 days, and other postoperative outcomes in long-term durable MCS patients. Findings may need further validation in larger studies.
Keywords: Frailty; Mechanical circulatory support; Outcomes; Rehospitalization; Survival
| Introduction | ▴Top |
The dawn of lasting continuous-flow pumps and inadequate organ donors for heart transplants have resulted in the utilization of mechanical circulatory support (MCS) in the care of subjects with end-stage heart failure [1]. Implantation of MCS such as left ventricular assist devices (LVAD) and total artificial heart (TAH) are acceptable treatment options for patients with advanced heart failure [2, 3]. MCS improves symptoms and quality of life of subjects with advanced heart failure by promoting sufficient organ perfusion and reinstating cardiac output [4, 5]. Frailty commonly coexists and is very prevalent among patients with advanced heart failure [6, 7]. Heart failure prevalence also increases six to seven times with worsening severity of frailty [8].
Frailty is a syndrome marked by increased vulnerability to adverse outcomes and reduced physiologic reserve [6]. Frailty has been linked with adverse effects such as the increased risk of death, poor compliance with treatment, increased risk of hospitalization in patients undergoing cardiac surgery, transcatheter aortic valve replacement, and heart failure [8, 9, 10, 11]. Because of the strong relationship between frailty and adverse health outcomes, there have been increased calls for routine application of frailty measurement for risk stratification of advanced heart failure patients [6]. Frailty status screening can enable prudent utilization of health care resources and helps with disease prognostication in addition to the appropriate selection of heart failure patients that are likely to benefit from advanced therapies such as placement of MCS [6].
Measurement of frailty status depends on mobility, functional level, strength, and cognitive status [12, 13]. Frailty status in patients undergoing durable MCS, especially TAH, is still understudied, and systematized measurement of frailty has not been wholly imbibed as routine in cardiovascular practice. Based on the above reasons, we aim to determine the association of frailty status with 1-year all-cause mortality, 30-day readmission, and other postoperative outcomes.
| Materials and Methods | ▴Top |
All patients undergoing placement of long-term MCS (LVAD or TAH) irrespective of the aim of surgery: bridge to transplant (BTT) or destination therapy (DT), between May 2018 and August 2020, were recruited for our study. We excluded three patients who did not have preoperative frailty status assessment. This study was conducted with the ethical standards of our institution on humans and the Helsinki Declaration. Our hospital’s Institutional Review Board reviewed and approved the study protocol.
Determination of frailty status
Frailty status was determined using the Fried frailty phenotype, and all the five domains were assessed before the implant ation of MCS. The five domains of Fried frailty phenotype assessed include self-reported exhaustion, grip strength, slow gait speed, reduced physical activity, and weight loss [14]. Patients were determined to be frail if the Fried frailty phenotype had three or more physical domains. Frailty was evaluated using Fried criteria as follows [14]. Unintentional weight loss was described as weight loss greater than 10 pounds or 5% of previous body weight in the last year [15]. Exhaustion was described as “yes” to any of these: “I perceive that everything that I did was an effort” and/or “I could not get going” at least 3 days per week. Criteria for slow gait speed were adjusted for height and gender and was defined as the time to walk 15 feet: female, height ≤ 1.59 m ≥ 7 s, height > 1.59 m ≥ 6 s; males, height ≤ 1.73 m ≥ 7 s, height >1.73 m ≥ 6 s [14]. Low physical activity was defined based on the standard algorithm of the shortened version of Minnesota leisure time activity (males expending less than 83 kcal/week and females spending less than 270). Grip strength was adjusted for body mass index and gender as defined by Fried et al [14].
Baseline variables
Baseline laboratory values: hemoglobin (g/dL), serum albumin (g/dL), total serum protein (g/dL) and prealbumin (mg/dL) was defined by values 24 hours prior to the implantation of MCS. Comorbidities of chronic obstructive pulmonary disease, sleep apnea, malignancy, depression, atrial fibrillation, cerebrovascular disease, and diabetes mellitus were defined by physician’s diagnoses obtained from the chart. Chronic kidney disease was described as an estimated glomerular filtration rate of less than 60 mL/min utilizing the renal disease equation [16]. The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profile score before MCS implantation varies from score 1 (critical cardiogenic shock) to 7 (advanced New York Heart Association class III symptoms) [17].
Outcome measures
The primary endpoint was 1-year post-intervention survival. The secondary endpoints include incidence of 30-day readmissions, gastrointestinal bleeding, neurological events (ischemic and hemorrhagic stroke), pump thrombosis, and driveline infection withins 12 months post-implantation.
Statistical analysis
The data were entered and stored into a secure RED cap database designated for the study. Dichotomous data were expressed as numbers (n) and percentages (%). Continuous data were expressed as mean ± standard deviation for normally distributed data or median (interquartile range) for non-normally distributed variables. Student’s t-tests or Mann-Whitney U tests were used to compare normally, or non-normally distributed continuous variables. Normality was tested using the Kolmogorov-Smirnov test. Pearson’s Chi-square or Fisher’s exact test was used to compare dichotomous variables depending on whether one or more expected cell frequency was less than 5. For survival estimates, survival time was defined as the time between the date of mechanical support implantation and the date of death or censoring (365 days from the time of MCS implant or date of heart transplant). The three patients that underwent heart transplantation within the following year were censored for estimation of survival estimates. The Kaplan-Meier curves estimated the association between frailty status and 1-year survival. The differences were examined with the log-rank test. Analysis was done using the Statistical Package for Social Sciences, version 28.0 (SPSS IBM., Armonk, NY, USA). A P values ≤ 0.05 was regarded as statistically significant.
| Results | ▴Top |
Out of the 46 patients who underwent MCS (TAH or LVAD) implantation at our institution, 32 (69%) patients were determined to be frail preoperatively according to the Fried frailty phenotype. Forty patients were implanted with LVAD (HeartWare ventricular assist device or Heartmate III), and six patients received SynCardia TAH. Twenty-six and 14 patients were implanted with Heartmate III and HeartWare ventricular assist device, respectively. The demographics, comorbidities, and laboratory parameters of the patients are presented in Table 1. Frailty was associated with lower serum albumin (g/dL) and left ventricular ejection fraction (%). Frail patients were more likely to have chronic kidney disease preoperatively.
 Click to view | Table 1. Baseline Demographics, Comorbidities, and Biochemical Parameters According to Fried Frailty Status |
Fried frailty phenotype and outcomes
Survival estimates were censored at 365 days from the date of durable MCS implantation and stratified by frailty status. There were no differences in overall survival between the frail group compared with the non-frail group on any durable MCS (Fig. 1), log-rank (Mantel-Cox), P = 0.165. In addition, there was no difference in survival between nonfrail and frail patients supported by LVAD only (Fig. 2), log-rank (Mantel-Cox), P = 0.233. No difference in survival estimates was observed between the two groups (frail and non-frail) supported by TAH (Fig. 3), log-rank (Mantel-Cox), P = 0.466.
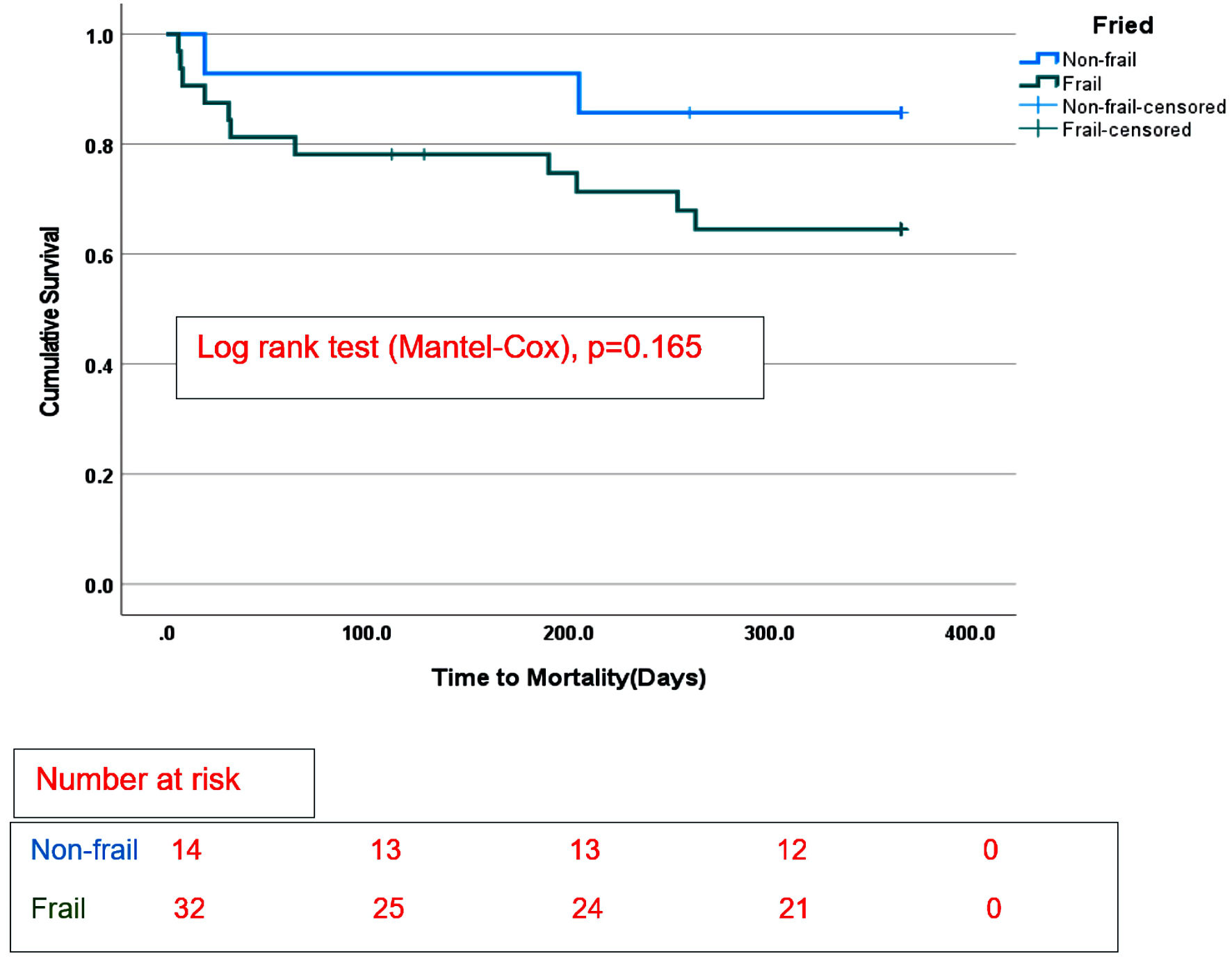 Click for large image | Figure 1. Survival categorized by frailty phenotype in the whole cohort with long-term mechanical circulatory support. |
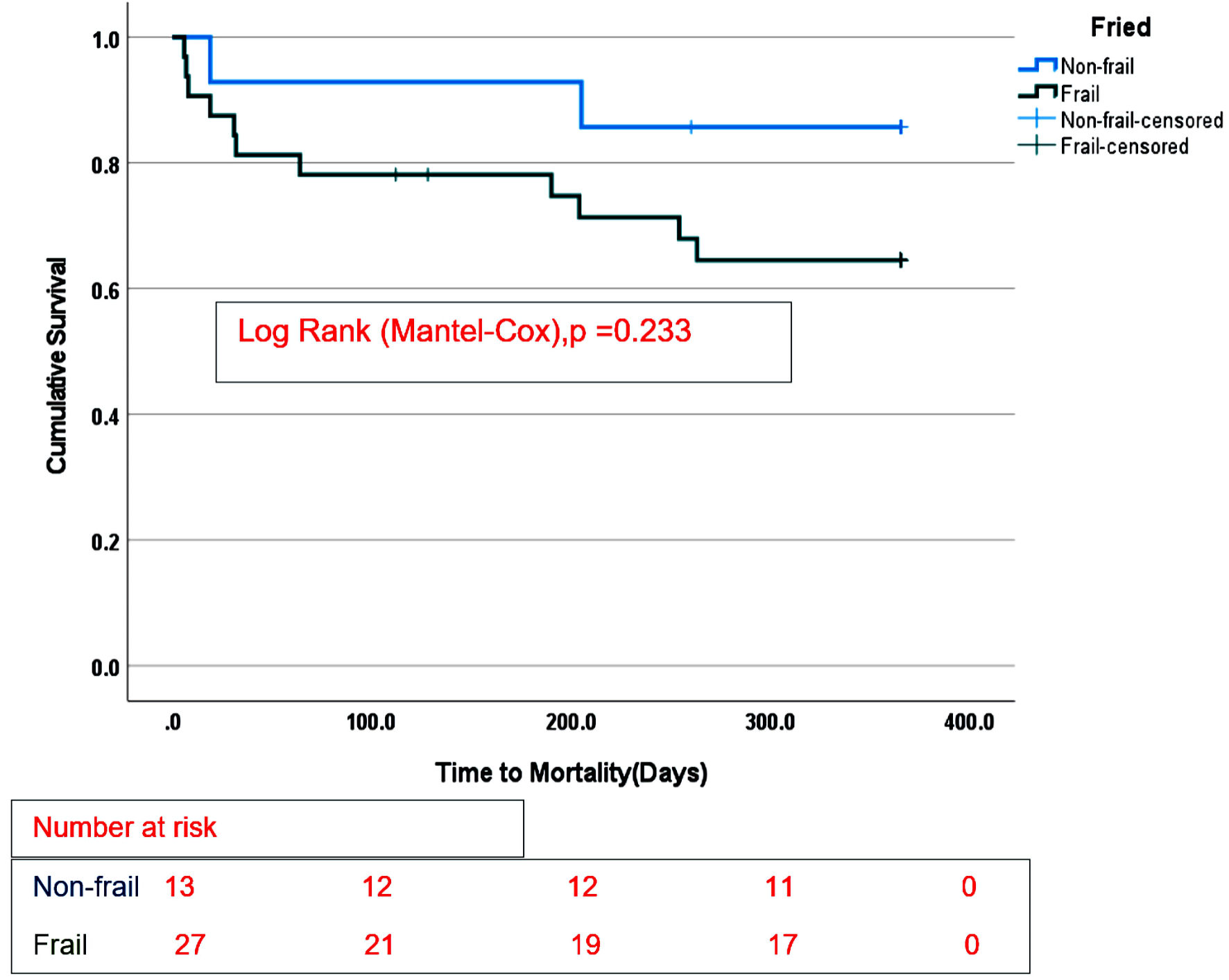 Click for large image | Figure 2. Survival stratified according to Fried frailty phenotype in LVAD recipients only. |
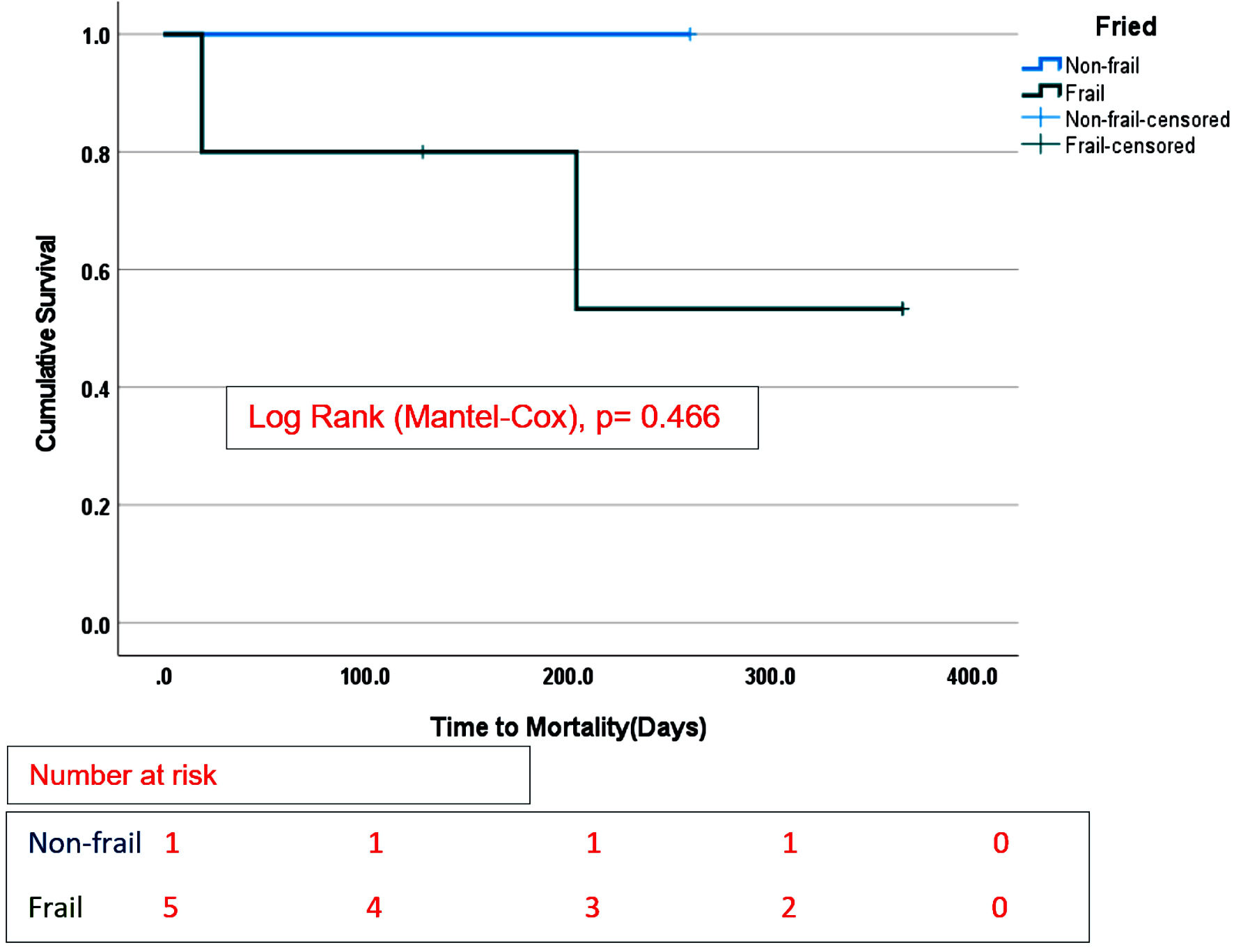 Click for large image | Figure 3. Overall survival stratified by Fried frailty phenotype in TAH recipients only. |
Eight patients died during the index hospitalization for placement of durable MCS, and they were excluded from the analysis of the 30-day readmission and other secondary endpoints.
There was no difference in 30-day readmission rates between the frail and non-frail groups supported by any durable MCS (P = 0.927). The results of other secondary end outcomes are shown in Table 2.
 Click to view | Table 2. Postoperative Outcomes Categorized by Fried Frailty Status |
| Discussion | ▴Top |
We performed this study to investigate if Fried frailty status has an impact on short-term and long-term outcomes in patients undergoing MCS placement. The principal findings of this study include: 1) There were no statistically significant differences in survival estimates between frail and non-frail patients supported by all durable MCS, LVAD, and TAH; 2) There were no differences in 30-day all-cause rehospitalization rates and other secondary outcomes.
Frailty is a complicated medical syndrome linked with biological aging compared to chronological age [18]. In addition, frailty and heart failure share a similar pathophysiological pathway of inflammation and abnormal hormonal regulation that makes patients prone to adverse outcomes on exposure to stressors such as MCS placement [19, 20].
There was no difference in 1-year survival estimates between the two groups of frail and non-frail patients on any durable MCS. But we noticed that frail patients trended towards lower survival rates at 1 year. In addition, seven out of eight patients that died during the index hospitalization for mechanical support placement were determined to be frail preoperatively. These observations may be explained by the inadequate number of deceased patients for analysis of the survival estimates.
Different studies have shown that preoperative frail patients have no statistically significant difference in long-term survival rates compared to non-frail patients after placement of mechanical support like our study. For example, Uzun et al reported no significant difference in all-cause mortality at 1 year after LVAD for the frail vs. non-frail subjects [20]. However, the study reported higher mortality rates (11% vs. 27%, P = 0.3) among non-frail patients. In another recent study of 107 BTT durable MCSs in Australia, there was no difference in mortality at 1 year between frail vs. non-frail patients (15.8% vs. 6.9%, P = 0.19) in patients supported with LVAD [21]. In another analysis, Joseph et al showed similar mortality rates between the two groups, with a mortality rate of 32% in frail patients versus 29% in non-frail patients (P = 1.00) [19].
In contrast to our findings, some studies have shown decreased survival in frail patients compared to non-frail after MCS implantation. For example, Dunlay et al reported a stepwise rise in 1-year mortality with increasing frailty defined by deficit index. In the study, the 1-year mortality rates of intermediate frail and frail patients (21.2% and 39.9%) were higher in comparison with non-frail patients (16.2%, P = 0.007) [22]. In a more extensive study of the INTERMACS database of 2,469 DT LVAD patients, the 1-year mortality rate was higher for patients assessed to be frail by the provider compared to non-frail (26.4 % vs. 18.9%, P = 0.01) [23]. Despite the conflicting results in the literature, frailty predisposes subjects to lower resistance against stressors such as mechanical support placement; these patients often take longer to recuperate, and subsequently stay longer in the hospital, predisposing them to poor outcomes such as deep venous thrombosis or hospital-acquired pneumonia [18]. Moreover, frailty creates an inflammatory milieu which increases oxidative stress by forming reactive oxygen species that creates an arrhythmogenic media predisposing subjects to ventricular arrhythmias and sudden death [24].
There were no differences in the age at the time of MCS implantation between frail and non-frail patients. Prior studies have reported a lack of relationship between chronological age and frailty in individuals undergoing durable MCS [19, 20, 25]. For instance, one of the studies reported that the frail group were younger and suggested that other studies avoid exclusion of more youthful patients from frailty assessment before MCS placement [19]. This present finding underscores the construct that frailty is a reflective of biological age rather than chronological age.
We hypothesized that 30-day readmission rates would be higher in frail patients after MCS implantation. Our findings showed similar 30-day readmission rates between the frail and non-frail groups after MCS implantation (40% vs. 39%, P = 0.927). A smaller previous study by Falls et al reported conflicting results. In the study, frail subjects were less likely to be rehospitalized within 30 days after discharge for LVAD placement compared to non-frail, (P = 0.033) [26]. Notwithstanding, 30-day readmissions are common, associated with an increased financial burden, and negatively influences the quality of life of these patients [27]. Furthermore, 30-day rehospitalizations has been previously associated with increased long-term mortality after LVAD support [27]. Therefore, the impact of frailty status in prognosticating early rehospitalization within 30 days needs further evaluation in larger multi-center studies.
Another finding in this study was the significantly lower serum albumin levels among preoperative frail patients. Interestingly, lower serum albumin levels have been linked with frailty in prior studies [28, 29]. Serum albumin levels are biomarkers for inflammation, nutritional state, and overall catabolic status [30-32]. In a previous study of the relation of preoperative serum albumin to survival in LVAD recipients, preoperative hypoalbuminemia (< 3.5 g/dL) was associated with poorer survival estimates at 3 months and 12 months after LVAD implantation [33]. Therefore, this finding in our study adds to the literature by underscoring the importance of preoperative nutritional assessment and nutrition intervention in addressing frailty in mechanical support recipients.
While MCS devices have been shown to improve survival and quality of life in selected patients, it has also been associated with complications such as cerebrovascular accidents, gastrointestinal bleeding, thrombosis, and device-associated infections [34]. We did not find any prior studies in the literature that studied postoperative outcomes of driveline infections, pump thrombosis and neurological events in relation to preoperative frailty status prior to implantation of long-term durable mechanical circulatory devices. Gastrointestinal bleeding is one of the most complications in recipients of durable MCS. The mechanisms involved in gastrointestinal bleeding are gastrointestinal tract angiodysplasia development, abnormal platelet aggregation, overutilization of anticoagulants, and acquired von Willebrand disease [35]. In addition, the proposed mechanism of thrombosis in frailty involves higher levels of inflammatory activation seen in frailty which can cause coagulopathy and thus, increase the risk of thrombotic events [36, 37] None of the secondary endpoints reached statistical significance despite the higher incidence of pump thrombosis and gastrointestinal bleeding in frail patients. A possible explanation for this may be due to the a small number of adverse events experienced in our study. The next step is to investigate these secondary outcomes with a larger sample size over a longer period than 1 year.
Our study has limitations that should be given due consideration. First, this is a single-center study, and outcomes were explored with 1 year of mechanical support. Because this is a single-center study, results may not be generalizable to the whole population of MCS patients. Due to our mid-volume center’s sample size, statistical tests may have been insufficiently powered to detect differences in 1-year survival rates.
Conclusions
Frailty status determined by Fried frailty phenotype did not impact our study’s short-term and long-term outcomes. However, as this is a single-center study and most of the studies found in the literature are also based on single-center findings, we recommend further multi-center studies to provide further details on the association of frailty status with postoperative outcomes of contemporary durable MCS.
Acknowledgments
None to declare.
Financial Disclosure
There was no funding or grant for this study.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
All subjects provided written informed consents.
Author Contributions
TA designed, analyzed, and drafted the manuscript. PC drafted and performed critical editing of the manuscript. RG supervised data collection and the preparation of the manuscript.
Data Availability
The data supporting the findings of our study are available within the manuscript.
Abbreviations
LVAD: left ventricular assist device; TAH: total artificial heart; LVEF: left ventricular ejection fraction; DM: diabetes mellitus; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; INTERMACS: Interagency Registry for Mechanically Assisted Circulatory Support; BTT: bridge to transplant; DT: destination therapy; MCS: mechanical circulatory support
| References | ▴Top |
- Gustafsson F, Rogers JG. Left ventricular assist device therapy in advanced heart failure: patient selection and outcomes. Eur J Heart Fail. 2017;19(5):595-602.
doi pubmed - Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53(15):e1-e90.
- Radovancevic B, Vrtovec B, Frazier OH. Left ventricular assist devices: an alternative to medical therapy for end-stage heart failure. Curr Opin Cardiol. 2003;18(3):210-214.
doi pubmed - Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241-2251.
doi pubmed - Estep JD, Starling RC, Horstmanshof DA, Milano CA, Selzman CH, Shah KB, Loebe M, et al. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: results from the ROADMAP study. J Am Coll Cardiol. 2015;66(16):1747-1761.
doi pubmed - Jha SR, Ha HS, Hickman LD, Hannu M, Davidson PM, Macdonald PS, Newton PJ. Frailty in advanced heart failure: a systematic review. Heart Fail Rev. 2015;20(5):553-560.
doi pubmed - Joyce E. Frailty in advanced heart failure. Heart Fail Clin. 2016;12(3):363-374.
doi pubmed - Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, et al. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53(8):1321-1330.
doi pubmed - Rockwood K, Howlett SE, MacKnight C, Beattie BL, Bergman H, Hebert R, Hogan DB, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2004;59(12):1310-1317.
doi pubmed - Green P, Woglom AE, Genereux P, Daneault B, Paradis JM, Schnell S, Hawkey M, et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. 2012;5(9):974-981.
doi pubmed - Afilalo J, Mottillo S, Eisenberg MJ, Alexander KP, Noiseux N, Perrault LP, Morin JF, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5(2):222-228.
doi pubmed - Rodriguez-Manas L, Feart C, Mann G, Vina J, Chatterji S, Chodzko-Zajko W, Gonzalez-Colaco Harmand M, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68(1):62-67.
doi pubmed - Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24.
doi pubmed - Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-156.
doi pubmed - Maurer MS, Horn E, Reyentovich A, Dickson VV, Pinney S, Goldwater D, Goldstein NE, et al. Can a left ventricular assist device in individuals with advanced systolic heart failure improve or reverse frailty? J Am Geriatr Soc. 2017;65(11):2383-2390.
doi pubmed - Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461-470.
doi pubmed - Stevenson LW, Pagani FD, Young JB, Jessup M, Miller L, Kormos RL, Naftel DC, et al. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant. 2009;28(6):535-541.
doi pubmed - Tse G, Gong M, Wong SH, Wu WKK, Bazoukis G, Lampropoulos K, Wong WT, et al. Frailty and clinical outcomes in advanced heart failure patients undergoing left ventricular assist device implantation: a systematic review and meta-analysis. J Am Med Dir Assoc. 2018;19(3):255-261.e251.
doi pubmed - Joseph SM, Manghelli JL, Vader JM, Keeney T, Novak EL, Felius J, Martinez SC, et al. Prospective assessment of frailty using the fried criteria in patients undergoing left ventricular assist device therapy. Am J Cardiol. 2017;120(8):1349-1354.
doi pubmed - Uzun HG, Simsek E, Engin C, Yagdi T, Karapolat H, Ozbaran M, Nalbantgil S. Relation between frailty and 1-year outcomes after implantation of a left ventricular assist device. Am J Cardiol. 2022;173:88-93.
doi pubmed - Muthiah K, Wilhelm K, Robson D, Raju H, Aili SR, Jha SR, Pierce R, et al. Impact of frailty on mortality and morbidity in bridge to transplant recipients of contemporary durable mechanical circulatory support. J Heart Lung Transplant. 2022;41(6):829-839.
doi pubmed - Dunlay SM, Park SJ, Joyce LD, Daly RC, Stulak JM, McNallan SM, Roger VL, et al. Frailty and outcomes after implantation of left ventricular assist device as destination therapy. J Heart Lung Transplant. 2014;33(4):359-365.
doi pubmed - Cooper LB, Hammill BG, Allen LA, Lindenfeld J, Mentz RJ, Rogers JG, Milano CA, et al. Assessing frailty in patients undergoing destination therapy left ventricular assist device: observations from interagency registry for mechanically assisted circulatory support. ASAIO J. 2018;64(1):16-23.
doi pubmed - Tse G, Yan BP, Chan YW, Tian XY, Huang Y. Reactive oxygen species, endoplasmic reticulum stress and mitochondrial dysfunction: the link with cardiac arrhythmogenesis. Front Physiol. 2016;7:313.
doi pubmed - Jha SR, Hannu MK, Newton PJ, Wilhelm K, Hayward CS, Jabbour A, Kotlyar E, et al. Reversibility of frailty after bridge-to-transplant ventricular assist device implantation or heart transplantation. Transplant Direct. 2017;3(7):e167.
doi pubmed - Falls C. Frailty in patients undergoing left ventricular assist device implantation. University of Kentucky College of Nursing. 2019.
- Gupta S, Cogswell RJ, Roy SS, Spratt JR, Liao KK, Martin CM, John R. Impact of 30 day readmission after left ventricular assist device implantation. ASAIO J. 2019;65(3):252-256.
doi pubmed - Smit E, Winters-Stone KM, Loprinzi PD, Tang AM, Crespo CJ. Lower nutritional status and higher food insufficiency in frail older US adults. Br J Nutr. 2013;110(1):172-178.
doi pubmed - Arques S, Roux E, Stolidi P, Gelisse R, Ambrosi P. Usefulness of serum albumin and serum total cholesterol in the prediction of hospital death in older patients with severe, acute heart failure. Arch Cardiovasc Dis. 2011;104(10):502-508.
doi pubmed - Don BR, Kaysen G. Poor nutritional status and inflammation: serum albumin: relationship to inflammation and nutrition. In: Proceedings of Seminars in dialysis. 2004;17(6):432-437.
doi pubmed - Fuhrman MP, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc. 2004;104(8):1258-1264.
doi pubmed - von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther. 2009;121(3):227-252.
doi pubmed - Kato TS, Kitada S, Yang J, Wu C, Takayama H, Naka Y, Farr M, et al. Relation of preoperative serum albumin levels to survival in patients undergoing left ventricular assist device implantation. Am J Cardiol. 2013;112(9):1484-1488.
doi pubmed - Potapov EV, Stepanenko A, Krabatsch T, Hetzer R. Managing long-term complications of left ventricular assist device therapy. Curr Opin Cardiol. 2011;26(3):237-244.
doi pubmed - Suarez J, Patel CB, Felker GM, Becker R, Hernandez AF, Rogers JG. Mechanisms of bleeding and approach to patients with axial-flow left ventricular assist devices. Circ Heart Fail. 2011;4(6):779-784.
doi pubmed - Phan HM, Alpert JS, Fain M. Frailty, inflammation, and cardiovascular disease: evidence of a connection. Am J Geriatr Cardiol. 2008;17(2):101-107.
- Van Epps P, Oswald D, Higgins PA, Hornick TR, Aung H, Banks RE, Wilson BM, et al. Frailty has a stronger association with inflammation than age in older veterans. Immun Ageing. 2016;13:27.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


