| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Review
Volume 14, Number 1, February 2023, pages 2-11
Assessment of Volume Status in Hospitalized Patients With Chronic Heart Failure
Joseph Racoa, c, Brandon Petersonb, Samer Muallemb
aDepartment of Internal Medicine, Penn State Milton S. Hershey Medical Center, Hershey, PA 17033, USA
bDepartment of Cardiology, Penn State Milton S. Hershey Medical Center, Hershey, PA 17033, USA
cCorresponding Author: Joseph Raco, Department of Internal Medicine, Penn State Milton S. Hershey Medical Center, Hershey, PA 17033, USA
Manuscript submitted October 15, 2022, accepted November 26, 2022, published online February 25, 2023
Short title: Assessment of Volume Status in HF Patients
doi: https://doi.org/10.14740/cr1434
- Abstract
- Introduction
- Clinical Features of Volume Overload
- Physical Examination
- Laboratory Abnormalities and Biometric Evaluation
- Imaging
- Invasive Evaluation
- Artificial Intelligence
- Conclusions
- References
| Abstract | ▴Top |
Assessment of volume status in hospitalized patients with heart failure is a critically important diagnostic skill that clinicians utilize frequently. However, accurate assessment is challenging and there is often significant inter-provider disagreement. This review serves as an appraisal of current methods of volume assessment amongst different categories of evaluation including patient history, physical exam, laboratory analysis, imaging, and invasive procedures. Within each category, this review highlights methods that are particularly sensitive or specific, or those that carry impactful positive or negative likelihood ratios. Utilization of the information that this review provides will allow clinicians to determine volume status of hospitalized heart failure patients more accurately and more precisely in order to provide appropriate and effective therapies.
Keywords: Volume status; Heart failure; Diagnostic; Volume assessment
| Introduction | ▴Top |
Accurate assessment of volume status among hospitalized patients with chronic heart failure (HF) is a critically important skill for physicians to master. Volume overload, or hypervolemia, is a frequent condition among these patients, especially those with increasing age, nutritional deficiencies, renal disease, or poor medication adherence [1]. Further, accurate assessment of volume status (i.e., euvolemia vs. hypo-/hypervolemia) often determines diagnostic and therapeutic strategies utilized by clinicians [2]. Many methods of volume analysis exist, ranging from invasive tests such as right heart catheterization, to noninvasive procedures such as echocardiography or point-of-care ultrasound (POCUS), to simple history and physical examination. Yet, determination of volume status frequently remains challenging and there is growing literature to suggest that many patients with HF are discharged without achieving complete clinical decongestion [2-4]. This review will serve as an assessment of the clinical, laboratory, physical exam, imaging, and procedural tools that we as providers have available for the assessment of volume status, while exploring the efficacy, difficulty, cost, and patient discomfort associated with each method of evaluation.
The pathophysiology of HF is complex, but the fundamental feature of this disease is cardiac compromise with a resultant decrease in cardiac index leading to compensatory expansion of circulating plasma volume (PV) [5-7]. As a result of impaired blood flow to the kidneys, there is increased activity of the renin-angiotensin-aldosterone system (RAAS) leading to renal retention of sodium and water, generating intravascular and interstitial volume expansion and redistribution [6, 8]. Initially, this compensatory response serves to maintain perfusion pressures, but over time becomes detrimental as the volume of fluid surpasses the existing intravascular capacity, hence the development of clinical congestion, extravascular fluid accumulation and increased cardiac afterload [8]. In order to blunt the effects of this pathologic volume expansion, a mainstay of HF therapy focuses on maintenance of clinical euvolemia, largely through the use of diuretics [2, 7]. Assessment of patients’ volume status is therefore a vital aspect of the management of HF patients. The intent of this review will be to provide a centralized reference to the various methods of volume assessment in an effort to determine fluid status more effectively and objectively in our patients.
| Clinical Features of Volume Overload | ▴Top |
Obtaining a focused clinical history remains the cornerstone of volume assessment. The majority of symptoms resulting from volume overload secondary to HF are nonspecific, including shortness of breath, dyspnea on exertion, weight gain, lethargy, fatigue, abdominal pain, loss of appetite, confusion, and dizziness [7, 9]. More specific symptoms include orthopnea (the feeling of increased shortness of breath while lying supine), and paroxysmal nocturnal dyspnea (PND, acute attacks of shortness of breath and coughing spells often awaking patients from sleep) [9]. Among these symptoms, orthopnea confers the highest positive likelihood ratio for hypervolemia [9]. Another atypical, often overlooked symptom of hypervolemia in HF patients is bendopnea, in which patients experience dyspnea when bending or leaning forward [10]. Recently, a systematic review and meta-analysis demonstrated that bendopnea is strongly associated with orthopnea, PND, New York Heart Association (NYHA) class IV symptoms and mortality in HF patients [11]. Additionally, close attention should be paid to risk factors for worsening HF including: hypertension, hyperlipidemia, recent myocardial infarction (MI), acute emotional stressors, worsening coronary artery disease (CAD), diabetes, and chronic kidney disease [5, 7]. Another important consideration in those with known HF is adherence to lifestyle modification and medications. Patients with HF are frequently on multiple cardiac medications, instructed to follow a low-salt diet and/or an oral fluid restriction with which poor compliance may predispose them to an acute exacerbation [7, 12]. While there is no one clinical feature of HF that is diagnostic of the disease, the patient’s history provides a clear first step for the clinician to determine an approximate pre-test probability of hypervolemia prior to further investigating with other methods of volume evaluation.
| Physical Examination | ▴Top |
Physical examination of a patient with suspected volume overload resulting from HF is a free, noninvasive, generally painless assessment that should be regularly performed prior to moving forward with any laboratory, imaging, or procedural methods of evaluation. As is often the case, the physical examination of a patient with suspected hypervolemia begins with carefully obtaining a patient’s vital signs. While no vital sign abnormalities are sensitive or specific for volume overload, tachycardia, hypertension, hypotension, tachypnea, and/or decreased peripheral oxygen saturation (SpO2) may be suggestive and abnormal vital signs can be indicative of severity of hypervolemia [7, 12].
Cardiac and lung auscultation remain an important component of the evaluative process for hypervolemia. The finding of a third heart sound (S3) on cardiac auscultation is associated with elevated intracardiac filling pressures [13]. A positive, reproducible S3 is associated with a strongly positive likelihood ratio (LR) for decreased ejection fraction (LR = 3.4 - 4.1), elevated left atrial pressures (LR = 3.9), and elevated B-type natriuretic peptide (BNP) levels (LR = 10.1), each of which are strong indicators of volume overload secondary to HF [13]. The findings of a fourth heart sound (S4) and/or valvular murmurs may also be associated with HF and hypervolemia, but less strongly [13]. Of note, a positive S3 may resolve after return to euvolemia, while valvular murmurs and S4 often will not [13].
Rales on lung auscultation are neither sensitive nor specific for hypervolemia secondary to HF, yet they remain an important clue to both the presence of cardiogenic pulmonary edema as well as response to treatment with diuretic therapy [7, 13]. A positive pulmonary auscultation finding of inspiratory and/or expiratory rales in a patient with known heart disease carries a LR of 2.1 for the presence of elevated left atrial pressure (again, a common manifestation of hypervolemia secondary to HF), and the dissipation of rales following therapy may be an indicator of resolving pulmonary edema [13]. Being that many patients with clinical volume overload complain of dyspnea, it is important to rule out pneumonia and other pulmonary parenchymal diseases prior to initiating therapy for HF [7]. Skillful auscultation of the lungs is an excellent, high-value and reliable method of narrowing one’s differential for dyspnea, as volume overload is not associated with wheezing, egophony, or changes in tactile fremitus, each of which would be suggestive of a separate or concomitant pulmonary process being present [13].
Another crucial aspect of the physical exam is an evaluation of jugular venous pressure (JVP). The assessment of the right jugular vein is often a difficult skill for clinicians to master, as it requires careful, delicate observation and additionally may be obscured in patients with especially large necks [13]. However, mastery of this skill can be helpful when examining for cardiac congestion. A JVP of > 8 cm of H2O is associated with a LR of 3.9 for the presence of elevated left atrial diastolic pressures, though a JVP of < 8 cm of H2O does not carry a significant negative LR for the same measure [13]. A carefully measured JVP has the potential to provide a strong positive predictive value for cardiac congestion by a skilled examiner. The diagnostic accuracy of JVP by experienced providers has been validated, with 85% of central venous pressure estimates based on JVP being within 4 cm of H2O of catheter-obtained measurements [13]. Further augmenting the jugular venous examination, one may assess the hepatojugular reflex (HJR, a maneuver designed to displace splanchnic venous blood towards the heart). This maneuver requires a skilled provider but in experienced hands provides a positive LR of 8.0 and a negative LR of 0.3 for the presence of elevated cardiac pressures [13].
Just as hypervolemic HF patents can develop increased hydrostatic pressures in their pulmonary vasculature contributing to pulmonary edema, the development of increased hydrostatic forces in systemic veins often leads to bilateral lower extremity edema [14]. While the presence or absence of bilateral leg pitting edema does not carry a significant positive or negative LR for the presence of HF, a reproducible presence of bilateral leg pitting edema does have a specificity of > 95% for the presence of HF in a patient with known or suspected cardiac compromise [13]. Clinicians should monitor the degree of peripheral edema during the course of hospitalization as a marker for response to diuretic therapy [7]. Diminished or absent peripheral pulses, in the absence of significant peripheral arterial disease, may reflect reduced cardiac output in decompensated congestive HF [15]. Relatedly, it is necessary to assess perfusion to distal extremities via observation of skin color and temperature. A bluish hue and/or cold temperature of the distal extremities reflects a lack of blood flow to distal organs, a concerning sign of decompensated HF [13, 15].
Another common method of fluid status evaluation is daily body weight (BW) monitoring. The simple premise for BW monitoring suggests that as patients accumulate fluid, their BW will increase, and providers can measure BW concurrently with diuretic therapy to assess therapy response. A growing body of literature supports the use of BW monitoring in the outpatient setting to guide diuretic regimens and assess risk for hospitalization for decompensated HF [16-18]. However, evidence for BW monitoring in hospitalized patients is lacking. Recently, a post hoc analysis was performed of the ASCEND-HF trial that examined BW changes during and after hospitalization. Patients with weight gain or minimal weight loss during hospitalization had significant associations with increased all-cause mortality, cardiovascular mortality and rehospitalization [19]. Significant weight loss during hospitalization was weakly associated with improvements in dyspnea and urine output [19]. Due to the strong infrastructure in place, ease of obtainment and low cost, the American College of Cardiology/American Heart Association (ACC/AHA) recommends monitoring of patient weights during hospital admissions for volume overload secondary to HF, supported by level C evidence [7]. Several physical examination findings and their sensitives, specificities, positive, and negative likelihood ratios can be found in Table 1 [9, 13].
 Click to view | Table 1. Sensitivity, Specificity and LRs of Various Physical Exam Findings for Detection of Elevated Intracardiac Pressures in Patients With Known or Suspected HF |
| Laboratory Abnormalities and Biometric Evaluation | ▴Top |
In patients with suspected decompensated HF, serum levels of N-terminal pro-B-type natriuretic peptide (NT-ProBNP) have become a routinely ordered study [5, 7, 20, 21]. The physiologic basis for this test is derived from the phenomenon that increased ventricular blood volume causes stretching of the ventricular myocytes, leading to the release of NT-ProBNP [22]. Serum NT-ProBNP level has been shown to correlate with the degree of volume overload and decompensated HF in patients [20, 21]. A baseline serum level of NT-ProBNP can be measured during periods of relative euvolemia, thereby providing clearer context to a particularly elevated value when the same patient is demonstrating signs and symptoms of hypervolemia [7]. The use of NT-ProBNP has become so impactful that multiple national cardiology societies have recommended the use of the marker to rule out the presence of decompensated HF when values are low [5, 22, 23]. The evidence for the use of NT-ProBNP for ruling out decompensated HF with volume overload is derived from the high sensitivity for the detection of decompensated HF [5, 23, 24]. NT-ProBNP is a low-cost lab test, which continues to become more affordable as testing becomes more standardized [25, 26]. Monitoring of NT-ProBNP has been demonstrated to be a high-value method of HF screening, as it holds the potential to decrease the number of further procedural tests and imaging studies performed, though this subject needs further study [25]. While the diagnostic utility of NT-ProBNP is promising, limitations of its use include false elevation in older patients, patients with atrial fibrillation, and patients with renal failure [20-22]. Alternatively, falsely low measurements may be obtained in obese patients [20-22].
Several other serum biomarkers have been assessed for utility in HF patients. These include troponin, galectin-3 and ST2 [27]. Among these, troponin is the most commonly used in clinical practice, though it is most well-studied and most commonly used in patients with concern for acute coronary syndromes [27]. The vast majority of patients with acute decompensated HF will have detectable troponin, so it has been proposed as a sensitive marker of HF decompensation [27, 28]. However, it is not directly associated with elevated intracardiac pressures and lacks specificity for hypervolemia, thus it is not recommended to be trended throughout hospitalization HF [28]. Galectin-3, a lectin product that is upregulated in response to stress or injury, has recently demonstrated association with worsening HF outcomes [29]. However, this marker has also not been found to be directly associated with hypervolemia [27, 29, 30]. Galectin-3 likely requires prospective study to demonstrate utility in guiding therapy, but it is currently given a grade IIb recommendation for use in patients with ACC stage C or D HF for the purposes of risk stratification and prognostication [7, 27]. ST2, the gene for interleukin-1 receptor-like 1 (IL1RL1), has long been known to be a marker of cardiomyocyte strain [31]. More recently, ST2 has demonstrated association with acute decompensated HF, HF mortality, and all-cause mortality independent of NT-ProBNP or troponin values [32, 33]. As with galectin-3 and troponin, ST2 has not been directly correlated to elevated filling pressures and thus, while each of these biomarkers carry useful prognostic information, their role in assessment of volume status remains limited [27]. Nonetheless, these markers, especially galectin-3 and ST2, represent opportunity for future study.
Another common lab analysis obtained in the evaluation of a patient with suspected volume overload is serum creatinine (Cr) and a derived estimated glomerular filtration rate (eGFR). Worsening renal function (WRF) occurs in > 90% of patients with decompensated HF, frequently resulting in acute kidney injury (AKI), defined as serum Cr > 1.5 × above baseline or > 0.3 mg/dL increase [34, 35]. The pathophysiology of cardiorenal syndrome (CRS) is remarkably complex and continually being further studied, but the current understanding indicates that WRF in patients with decompensated HF is a multi-systemic process occurring as a pathologic response to cardiac and intravascular congestion [34, 35]. In effort to reduce this congestion, CRS is generally treated with diuretics with improvement in serum Cr [35]. However, current data are mixed regarding the prognostic utility of WRF in hypervolemic patients and whether or not providers should be targeting a return to baseline serum Cr [35, 36]. As such, no strong recommendation has been made for or against the monitoring of serum Cr in HF patients admitted to the hospital, but with the low cost associated with this lab test, monitoring remains standard practice and appears to be safe [23, 35, 36].
As stated previously, PV expansion is a hallmark of worsening HF. Several methods of PV measurement exist, but direct monitoring with radioisotope assays is invasive and are expensive [37, 38]. As such, a method of estimating plasma volume status (PVS) noninvasively has long been sought after, with multiple estimation equations studied in recent years [37, 38]. Ling et al studied a proposed PVS equation in 2014 based upon actual plasma volume (aPV) and ideal plasma volume (iPV) in order to derive a minimally-invasive, cost-effective method of true PVS [37]. The study concluded that their calculated PVS accurately reflected patient’s degree of volume overload and was independently correlated to patient outcomes [37]. The proposed equation may be found here (Supplemental Material 1, www.cardiologyres.org). In 2018, Fudim et al conducted a review of estimated PV calculations and their correlation to aPV, concluding that calculated estimates of PV demonstrate limited association with aPV, though they may retain prognostic utility [38]. Recently, Yaranov et al investigated total blood volume (TBV), PV and red blood cell volume (RBCV) monitoring via the CardioMEMS implantable pulmonary arterial pressure monitor in a 20-patient cohort [39]. Findings indicated that pulmonary arterial and intracardiac pressures, which are traditionally relied upon measures of volume status in HF patients, may not be representative of TBV in select patients and phenotypes [39]. Future research is required to fully elucidate the nuanced balance of pressure-volume phenotypes in HF patients and invasive volume-guided phenotyping may hold promise in improving clinical outcomes with respect to achieving euvolemia [39]. Invasive hemodynamic monitoring is discussed further below.
Bioelectrical impedance analysis (BIA) has been studied as a rapid, noninvasive and inexpensive method for the evaluation of volume status [40-45]. Bioimpedance analysis utilizes electrical currents from superficially-placed electrodes to assess fluid composition across a given area, operating on the theory that fluid will more efficiently conduct electricity than solid tissue [40]. Both whole body or segmental BIA can be measured depending on placement of electrodes and may provide an objective assessment of the fluid content within a given area [40]. Parrinello et al first established the reliability of BIA in 2008 when it was found to correlate well with BNP and diagnosis of decompensated HF [40]. Since this time, several retrospective and prospective analyses have demonstrated utility of BIA as an independent prognostic marker for patients with HF and specific parameters have been suggested to have clinical utility to indicate successful decongestion [42, 44, 45]. While not yet incorporated into routine clinical practice to the degree of serum biomarkers such as NT-ProBNP, BIA has potential to augment clinician judgment with regards to volume assessment [43, 45]. Prospective, controlled such as the SCALE-HF trials using BIA to guide clinical decision making in HF patients are currently underway [46, 47]. This noninvasive, objective modality of evaluation holds considerable promise in improving clinician ability to accurately ascertain congestion status.
| Imaging | ▴Top |
Dyspnea is a frequent chief complaint of patients with volume overload secondary to HF, and as such, chest X-rays (CXRs) are frequently the first-line imaging study obtained upon initial patient evaluation [48]. While the specificity of CXR for diagnosis of decompensated HF is poor, there is value in using this study to rule out alternative cardiopulmonary causes of dyspnea such as chronic obstructive pulmonary disease (COPD) and pneumonia [48-51]. Although no CXR findings are diagnostic of HF, there are several suggestive findings that may further suggest a patient’s clinical diagnosis. These findings include upper lung zone flow redistribution, lung interstitial or alveolar edema, pleural effusions, pulmonary vessel cephalization, and cardiomegaly [48, 51]. Advantages of CXR in the initial evaluation of suspected HF patients include the relative ease of interpretation as well as cost-effectiveness, especially when compared to specialized imaging studies such as echocardiography, computed tomography (CT), and magnetic resonance imaging (MRI) [48]. Finally, having documentation of CXRs in the electronic medical record consisting of both baseline and volume overloaded states may be helpful for future providers during exacerbations of clinical status [48]. The American College of Radiology therefore recommends obtaining a CXR during the evaluation of all patients with dyspnea of suspected cardiac origin [50]. A number of CXR findings and their associated sensitivities, specificities, and positive and negative predictive values are outlined in Table 2 [48, 51]. As stated previously, CXR findings alone are insufficient for diagnosis of HF.
 Click to view | Table 2. Sensitivity, Specificity and PPV of Various CXR Findings for Diagnosis of HF |
Due to its high sensitivity and specificity for the diagnosis of cardiac congestion, transthoracic echocardiography (TTE) is recommended by multiple international radiologic and cardiovascular societies as an essential initial investigation in the diagnosis of volume overload secondary to HF [23, 50, 52]. This recommendation is further supported by TTE’s relatively low cost, ease of administration, patient comfortability, and lack of radiation exposure, as well as its ability to assess hemodynamics, cardiac structure and function [52-54]. With experienced providers and new echocardiographic technology including three-dimensional (3D) capability and Doppler assessments, TTE has the ability to accurately assess left ventricular systolic and diastolic function, valvular competency, left atrial volume, right atrial pressure estimates, and regional wall motion abnormalities [52, 54]. Further, TTE can be performed at rest or as part of a cardiac stress evaluation, providing essential data regarding cardiac function and exercise tolerance [52, 53]. With the versatility of this study, it is reasonable to suggest that TTE is the gold standard, noninvasive diagnostic study for both HF and volume overload states. However, in advanced HF states, echocardiographic findings may be insufficient and require further evaluation with more invasive procedures for accurate and reliable assessment of volume status, intracardiac filling pressures and cardiac output [52-55].
It is important to note that a formal echocardiographic evaluation, performed and interpreted by a highly trained provider is required in order to achieve the complete benefits of a TTE study [53]. However, as ultrasound technology continues to evolve, the use of POCUS for basic cardiologic assessment by general medical providers has become increasingly common and represents an exciting and innovative area of contemporary research [56-58]. The routine use of ultrasound investigation of cardiopulmonary measures at the bedside has drawn both support and cautious criticism [56, 57, 59]. A number of small, randomized trials have evaluated the utility of POCUS as a tool for improving efficiency and accuracy of diagnosis of a variety of intrathoracic conditions including respiratory failure and cardiopulmonary congestion from HF, the results of which have been in favor of its use [60-63]. There is some evidence to suggest that demonstration of pulmonary B-lines on POCUS and measurement of inferior vena cava (IVC) diameters may outperform traditional objective measures of volume assessment including NT-ProBNP level and CXR findings [64, 65]. Expert opinion currently supports the use of POCUS as an augmentation to comprehensive volume status examination, which has been supported by consensus statements and recommendations by the American Society of Echocardiography and the American College of Physicians [66, 67]. As the technology and practice of POCUS continues to advance, it may be incorporated into standard of practice for evaluation of patients with hypervolemia or dyspnea suspected to be related to HF.
| Invasive Evaluation | ▴Top |
While a diagnosis of volume overload secondary to HF can be made through a combination of careful clinical history taking and examination along with use, when indicated, of noninvasive imaging such as echocardiography, there are several instances in which invasive hemodynamic assessment is appropriate. Indications for these invasive strategies include confirmation of pulmonary hypertension suggested on echocardiography, evaluation of persistent hemodynamic instability, investigation of treatment resistance, worsening of renal function with therapy, and further evaluation in patients being considered for heart transplant or mechanical circulatory support [7]. Advantages of these techniques include their high diagnostic precision and the ability to directly measure parameters that would otherwise require derived or inferred values, though their utility is balanced by their potential for procedural complications, associated patient discomfort and cost when compared with previously described methods of evaluation [7]. As such, noninvasive, low-cost and readily-available methods of volume assessment, such as those discussed previously, remain the cornerstone of evaluation in HF patients, which is reflected in Figure 1.
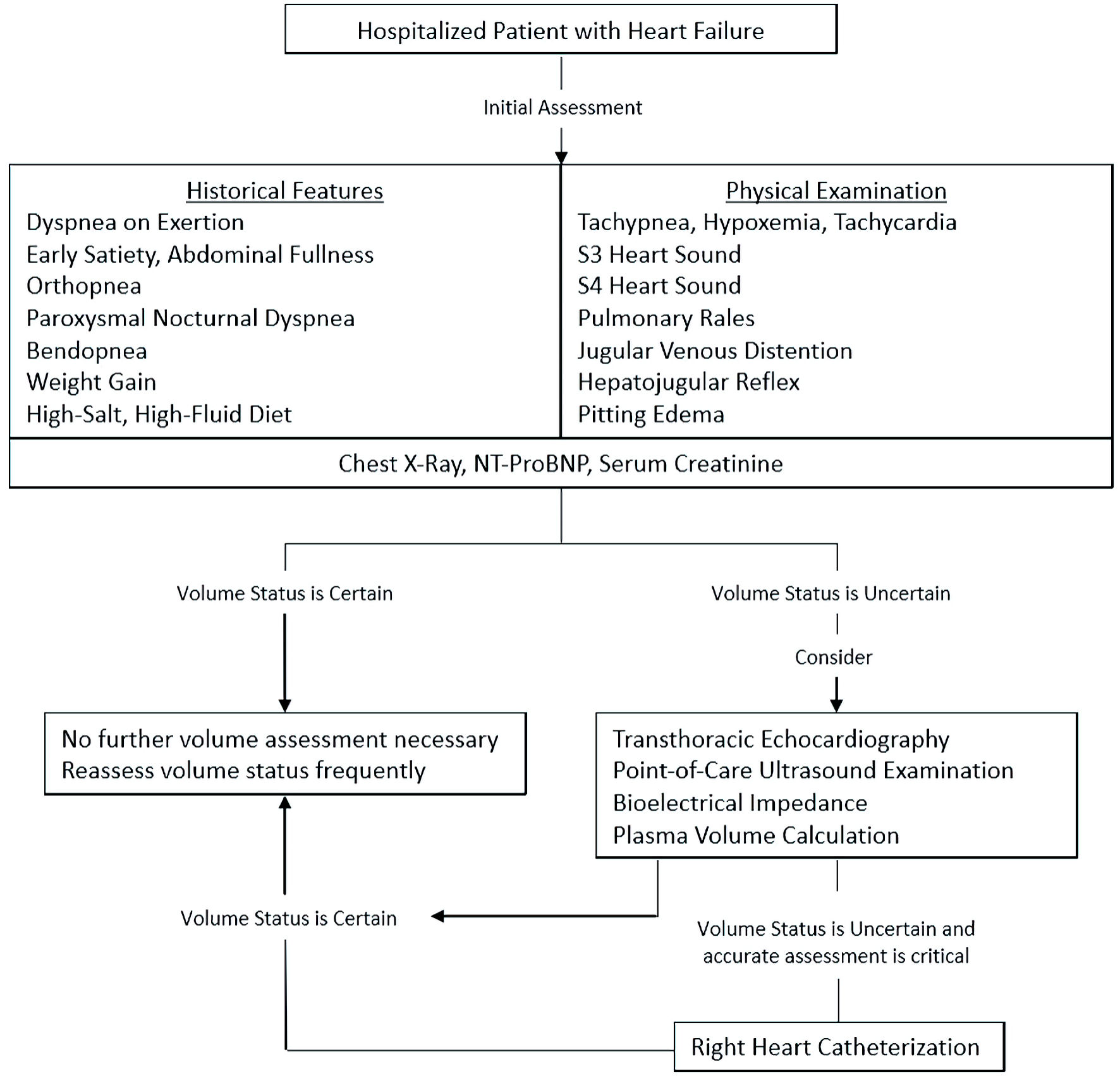 Click for large image | Figure 1. Suggested diagnostic approach for volume status assessment in hospitalized patients with HF. HF: heart failure; NT-ProBNP: N-terminal pro-B-type natriuretic peptide. |
The most common invasive technique for assessment of intravascular volume status in HF patients is the right-heart catheterization (RHC) with a pulmonary artery catheter [7, 68, 69]. RHC allows providers the ability to directly record intracardiac and intrapulmonary pressures to the point of the pulmonary capillaries, with right atrial pressures (RAP) or central venous pressures (CVP), right ventricular pressures (RVP), mean pulmonary artery pressures (mPAP), and pulmonary capillary wedge pressures (PCWP) being the most commonly utilized datapoints [68-70]. A recording of elevated right heart and pulmonary arterial pressures provide valuable information for both the presence and extent of intravascular congestion, giving care teams direct insight into intrathoracic hemodynamics [68, 70]. Recently, Ma et al studied the association of a “cardiac congestion index” in which the investigators divided post-RHC patients into two groups: those with a RAP + PCWP of < 30 mm Hg (low) and those with a RAP + PCWP of > 30 mm Hg (high) [71]. It was found that patients with a low cardiac congestion index had a significantly lower 6-month mortality rate, rehospitalization rate and transplantation rate, indicating that a cardiac congestion index has significant prognostic utility [71]. While further study is required in order to determine if this is a viable strategy for influencing treatment of volume overloaded HF patients, the authors concluded that this congestion index has the potential to indicate the need for more aggressive interventions for patients in the high index subgroup with the possibility of decreasing mortality in this cohort [71]. While it is clear that in certain instances, RHC gives critical information to providers, the invasiveness of the procedure has led to the ACC/AHA (2013), as well as the European Society of Cardiology (2021) to recommend against routine RHC in patients with HF, except for those whose fluid status remains uncertain despite noninvasive evaluation, select patients in cardiogenic shock, and patients who are refractory to diuretic therapy [7, 28, 70].
It is worth mentioning that hemodynamic monitoring of left-sided cardiac pressures via left heart catheterization (LHC) procedures may also play a role in the assessment of volume overloaded HF patients by allowing for measurement of left ventricular end diastolic pressure (LVEDP) [7, 70]. However, the clinical indications of this procedure are significantly narrower than those for RHC and it is higher risk than RHC due to the need for arterial access [70]. LHC’s limited utility for intravascular volume assessment makes the procedure fall beyond the scope of this review.
In recent years, several implantable hemodynamic monitors (IHMs) have been produced in an effort to provide continuous hemodynamic measurement without subsequent cardiac catheterization procedures [23, 72]. These devices include wireless pulmonary artery pressure monitors (CardioMEMS), thoracic impedance measurements (OptiVol), implantable RVP monitoring systems (Chronicle), implanted cardiac defibrillators (ICD), dual-chamber pacemakers and cardiac resynchronization therapy devices (CRT) [72-75]. It is hypothesized that remote monitoring of HF patients may be more accurate than patient-reported changes in weight or subjective dyspnea for ambulatory monitoring of patients susceptible to decompensated HF [72]. The physiologic parameters measured by the devices can be delivered to either a clinician or the patient to guide management decisions [72]. Each of these implantable devices have been studied in large, randomized controlled trials with mixed evidence for reductions in HF hospitalizations, cardiovascular mortality and all-cause mortality [73-75]. A recent systematic review and meta-analysis of remote monitoring for HF found that while these devices have not significantly improved previously mentioned endpoints, RV and/or pulmonary pressure monitoring may reduce HF hospitalizations [72]. Several other IHMs are currently in development and hold promise for improving the care delivered to HF patients [72].
| Artificial Intelligence | ▴Top |
Despite the abundance of information available to the modern-day clinician, successful restoration of euvolemia in HF patients remains challenging, as many patients hospitalized with acute decompensated HF are discharged with persistent volume overload [7, 23]. Numerous tools remain in development to aid clinician assessment. One such consideration is the use of automated intelligence (AI) to assist with provider discretion. Recently, Celik et al, in the ART-IN-HF study, evaluated the plausibility of AI assisted CXR interpretation and concluded that AI may hold promise in new-diagnosis of HF [76]. Further, Yasmin et al performed a review on the role of AI in modern HF diagnosis and treatment, highlighting the potential benefits that AI may offer in coming years [77].
| Conclusions | ▴Top |
The various clinical, laboratory, imaging, and procedural tools that providers have for the assessment of volume status in HF patients are continually evolving. This has led to a drastic expansion of data points that we as clinicians have at our discretion for patient evaluation. Though it remains critical that our evaluations are built upon detailed and thorough history taking, physical examination and noninvasive imaging techniques, considerable progress has been made with biomarkers, remote monitoring devices, bioimpedance analysis and invasive monitoring that providers must remain familiar with. Utilization and mastery of a multi-modal approach to volume assessment may improve rates of successful decongestion and restoration of euvolemia, conferring better health and reduced hospitalizations for our patients with HF.
| Supplementary Material | ▴Top |
Suppl 1. Formula to calculate aPV.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
Joseph Raco wrote the original version of the manuscript and performed all background literature review, created all tables, figures and citations, implemented edits from Dr. Muallem and Dr. Peterson, and submitted the manuscript. Brandon Peterson provided many edits to the original and edited manuscripts, provided expert opinion and general guidance. Samer Muallem provided several edits to the original manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Chang PP, Wruck LM, Shahar E, Rossi JS, Loehr LR, Russell SD, Agarwal SK, et al. Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005-2014): ARIC study community surveillance. Circulation. 2018;138(1):12-24.
doi pubmed - Strobeck JE, Feldschuh J, Miller WL. Heart failure outcomes with volume-guided management. JACC Heart Fail. 2018;6(11):940-948.
doi pubmed - Lala A, McNulty SE, Mentz RJ, Dunlay SM, Vader JM, AbouEzzeddine OF, DeVore AD, et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from diuretic optimization strategy evaluation in acute decompensated heart failure (DOSE-AHF) and cardiorenal rescue study in acute decompensated heart failure (CARESS-HF). Circ Heart Fail. 2015;8(4):741-748.
doi pubmed - Mullens W, Dauw J, Martens P, Verbrugge FH, Nijst P, Meekers E, Tartaglia K, et al. Acetazolamide in acute decompensated heart failure with volume overload. N Engl J Med. 2022;387(13):1185-1195.
doi pubmed - Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, Anker SD, et al. Universal definition and classification of heart failure: a report of the heart failure society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;23(3):352-380.
doi pubmed - Miller WL. Fluid volume overload and congestion in heart failure: time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail. 2016;9(8):e002922.
doi pubmed - Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr., Drazner MH, Fonarow GC, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810-1852.
doi pubmed - Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 2016;12(10):610-623.
doi pubmed - Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294(15):1944-1956.
doi pubmed - Thibodeau JT, Turer AT, Gualano SK, Ayers CR, Velez-Martinez M, Mishkin JD, Patel PC, et al. Characterization of a novel symptom of advanced heart failure: bendopnea. JACC Heart Fail. 2014;2(1):24-31.
doi pubmed - Pranata R, Yonas E, Chintya V, Alkatiri AA, Budi Siswanto B. Clinical significance of bendopnea in heart failure-Systematic review and meta-analysis. Indian Heart J. 2019;71(3):277-283.
doi pubmed - Houston BA, Kalathiya RJ, Kim DA, Zakaria S. Volume overload in heart failure: an evidence-based review of strategies for treatment and prevention. Mayo Clin Proc. 2015;90(9):1247-1261.
doi pubmed - McGee S. Chapter 1 - What Is Evidence-Based Physical Diagnosis? Evidence-based physical diagnosis. Elsevier. 2018.
doi - Whiting E, McCready ME. Pitting and non-pitting oedema. Med J Aust. 2016;205(4):157-158.
doi pubmed - Mitchell GF, Tardif JC, Arnold JM, Marchiori G, O'Brien TX, Dunlap ME, Pfeffer MA. Pulsatile hemodynamics in congestive heart failure. Hypertension. 2001;38(6):1433-1439.
doi pubmed - Goldberg LR, Piette JD, Walsh MN, Frank TA, Jaski BE, Smith AL, Rodriguez R, et al. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: the Weight Monitoring in Heart Failure (WHARF) trial. Am Heart J. 2003;146(4):705-712.
doi pubmed - Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation. 2007;116(14):1549-1554.
doi pubmed - Lynga P, Persson H, Hagg-Martinell A, Hagglund E, Hagerman I, Langius-Eklof A, Rosenqvist M. Weight monitoring in patients with severe heart failure (WISH). A randomized controlled trial. Eur J Heart Fail. 2012;14(4):438-444.
doi pubmed - Ambrosy AP, Cerbin LP, Armstrong PW, Butler J, Coles A, DeVore AD, Dunlap ME, et al. Body weight change during and after hospitalization for acute heart failure: patient characteristics, markers of congestion, and outcomes: findings from the ASCEND-HF trial. JACC Heart Fail. 2017;5(1):1-13.
doi pubmed - Taub PR, Daniels LB, Maisel AS. Usefulness of B-type natriuretic peptide levels in predicting hemodynamic and clinical decompensation. Heart Fail Clin. 2009;5(2):169-175.
doi pubmed - McKie PM, Burnett JC, Jr. NT-proBNP: the gold standard biomarker in heart failure. J Am Coll Cardiol. 2016;68(22):2437-2439.
doi pubmed - Chopra S, Cherian D, Verghese PP, Jacob JJ. Physiology and clinical significance of natriuretic hormones. Indian J Endocrinol Metab. 2013;17(1):83-90.
doi pubmed - Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891-975.
doi pubmed - Taylor KS, Verbakel JY, Feakins BG, Price CP, Perera R, Bankhead C, Pluddemann A. Diagnostic accuracy of point-of-care natriuretic peptide testing for chronic heart failure in ambulatory care: systematic review and meta-analysis. BMJ. 2018;361:k1450.
doi pubmed - Ferrandis MJ, Ryden I, Lindahl TL, Larsson A. Ruling out cardiac failure: cost-benefit analysis of a sequential testing strategy with NT-proBNP before echocardiography. Ups J Med Sci. 2013;118(2):75-79.
doi pubmed - Bugge C, Sether EM, Pahle A, Halvorsen S, Sonbo Kristiansen I. Diagnosing heart failure with NT-proBNP point-of-care testing: lower costs and better outcomes. A decision analytic study. BJGP Open. 2018;2(3):bjgpopen18X101596.
doi pubmed - de Boer RA, Daniels LB, Maisel AS, Januzzi JL, Jr. State of the Art: Newer biomarkers in heart failure. Eur J Heart Fail. 2015;17(6):559-569.
doi pubmed - McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, et al. [2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC]. G Ital Cardiol (Rome). 2022;23(4 Suppl 1):e1-e127.
- Gullestad L, Ueland T, Kjekshus J, Nymo SH, Hulthe J, Muntendam P, Adourian A, et al. Galectin-3 predicts response to statin therapy in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA). Eur Heart J. 2012;33(18):2290-2296.
doi pubmed - Wu C, Lv Z, Li X, Zhou X, Mao W, Zhu M. Galectin-3 in predicting mortality of heart failure: a systematic review and meta-analysis. Heart Surg Forum. 2021;24(2):E327-E332.
doi pubmed - Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106(23):2961-2966.
doi pubmed - Rehman SU, Mueller T, Januzzi JL, Jr. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52(18):1458-1465.
doi pubmed - Pascual-Figal DA, Manzano-Fernandez S, Boronat M, Casas T, Garrido IP, Bonaque JC, Pastor-Perez F, et al. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: complementary role for risk stratification in acutely decompensated heart failure. Eur J Heart Fail. 2011;13(7):718-725.
doi pubmed - Kumar U, Wettersten N, Garimella PS. Cardiorenal syndrome: pathophysiology. Cardiol Clin. 2019;37(3):251-265.
doi pubmed - Kazory A. The congestion-creatinine interplay in acute heart failure: time to move up to the next level. Am J Med. 2020;133(3):259-260.
doi pubmed - Brisco MA, Zile MR, Hanberg JS, Wilson FP, Parikh CR, Coca SG, Tang WH, et al. Relevance of changes in serum creatinine during a heart failure trial of decongestive strategies: insights from the DOSE trial. J Card Fail. 2016;22(10):753-760.
doi pubmed - Ling HZ, Flint J, Damgaard M, Bonfils PK, Cheng AS, Aggarwal S, Velmurugan S, et al. Calculated plasma volume status and prognosis in chronic heart failure. Eur J Heart Fail. 2015;17(1):35-43.
doi pubmed - Fudim M, Miller WL. Calculated estimates of plasma volume in patients with chronic heart failure-comparison with measured volumes. J Card Fail. 2018;24(9):553-560.
doi pubmed - Yaranov DM, Jefferies JL, Silver MA, Burkhoff D, Rao VN, Fudim M. Discordance of pressure and volume: potential implications for pressure-guided remote monitoring in heart failure. J Card Fail. 2022;28(5):870-872.
doi pubmed - Parrinello G, Paterna S, Di Pasquale P, Torres D, Fatta A, Mezzero M, Scaglione R, et al. The usefulness of bioelectrical impedance analysis in differentiating dyspnea due to decompensated heart failure. J Card Fail. 2008;14(8):676-686.
doi pubmed - Genot N, Mewton N, Bresson D, Zouaghi O, Francois L, Delwarde B, Kirkorian G, et al. Bioelectrical impedance analysis for heart failure diagnosis in the ED. Am J Emerg Med. 2015;33(8):1025-1029.
doi pubmed - Sakaguchi T, Yasumura K, Nishida H, Inoue H, Furukawa T, Shinouchi K, Miura H, et al. Quantitative assessment of fluid accumulation using bioelectrical impedance analysis in patients with acute decompensated heart failure. Circ J. 2015;79(12):2616-2622.
doi pubmed - Scicchitano P, Massari F. The role of bioelectrical phase angle in patients with heart failure. Rev Endocr Metab Disord. 2022.
doi pubmed - Scicchitano P, Ciccone MM, Iacoviello M, Guida P, De Palo M, Potenza A, Basile M, et al. Respiratory failure and bioelectrical phase angle are independent predictors for long-term survival in acute heart failure. Scand Cardiovasc J. 2022;56(1):28-34.
doi pubmed - Smeets CJP, Lee S, Groenendaal W, Squillace G, Vranken J, De Canniere H, Van Hoof C, et al. The added value of in-hospital tracking of the efficacy of decongestion therapy and prognostic value of a wearable thoracic impedance sensor in acutely decompensated heart failure with volume overload: prospective cohort study. JMIR Cardio. 2020;4(1):e12141.
doi pubmed - Eckstein J. Smart SCALEs with bioimpedance analysis for treatment guidance in decompensated heart failure (SCALE HF).
- Devore A. Surveillance and alert-based multiparameter monitoring to reduce worsening heart failure events (SCALE-HF 1).
- Fonseca C, Mota T, Morais H, Matias F, Costa C, Oliveira AG, Ceia F, et al. The value of the electrocardiogram and chest X-ray for confirming or refuting a suspected diagnosis of heart failure in the community. Eur J Heart Fail. 2004;6(6):807-812.
doi pubmed - Kennedy S, Simon B, Alter HJ, Cheung P. Ability of physicians to diagnose congestive heart failure based on chest X-ray. J Emerg Med. 2011;40(1):47-52.
doi pubmed - Expert Panel on Cardiac I, Vogel-Claussen J, Elshafee ASM, Kirsch J, Brown RKJ, Hurwitz LM, Javidan-Nejad C, et al. ACR appropriateness criteria((R)) dyspnea-suspected cardiac origin. J Am Coll Radiol. 2017;14(5S):S127-S137.
doi pubmed - Mueller-Lenke N, Rudez J, Staub D, Laule-Kilian K, Klima T, Perruchoud AP, Mueller C. Use of chest radiography in the emergency diagnosis of acute congestive heart failure. Heart. 2006;92(5):695-696.
doi pubmed - Marwick TH. The role of echocardiography in heart failure. J Nucl Med. 2015;92(Suppl 4):31S-38S.
doi pubmed - Pastore MC, Mandoli GE, Aboumarie HS, Santoro C, Bandera F, D'Andrea A, Benfari G, et al. Basic and advanced echocardiography in advanced heart failure: an overview. Heart Fail Rev. 2020;25(6):937-948.
doi pubmed - Melillo E, Masarone D, Oh JK, Verrengia M, Valente F, Vastarella R, Ammendola E, et al. Echocardiography in advanced heart failure for diagnosis, management, and prognosis. Heart Fail Clin. 2021;17(4):547-560.
doi pubmed - D'Alto M, Romeo E, Argiento P, D'Andrea A, Vanderpool R, Correra A, Bossone E, et al. Accuracy and precision of echocardiography versus right heart catheterization for the assessment of pulmonary hypertension. Int J Cardiol. 2013;168(4):4058-4062.
doi pubmed - Smallwood N, Dachsel M. Point-of-care ultrasound (POCUS): unnecessary gadgetry or evidence-based medicine? Clin Med (Lond). 2018;18(3):219-224.
doi pubmed - Kovell LC, Ali MT, Hays AG, Metkus TS, Madrazo JA, Corretti MC, Mayer SA, et al. Defining the role of point-of-care ultrasound in cardiovascular disease. Am J Cardiol. 2018;122(8):1443-1450.
doi pubmed - Johri AM, Durbin J, Newbigging J, Tanzola R, Chow R, De S, Tam J. Cardiac point-of-care ultrasound: state-of-the-art in medical school education. J Am Soc Echocardiogr. 2018;31(7):749-760.
doi pubmed - Blanco P, Volpicelli G. Common pitfalls in point-of-care ultrasound: a practical guide for emergency and critical care physicians. Crit Ultrasound J. 2016;8(1):15.
doi pubmed - Ben-Baruch Golan Y, Sadeh R, Mizrakli Y, Shafat T, Sagy I, Slutsky T, Kobal SL, et al. Early Point-of-Care Ultrasound Assessment for Medical Patients Reduces Time to Appropriate Treatment: A Pilot Randomized Controlled Trial. Ultrasound Med Biol. 2020;46(8):1908-1915.
doi pubmed - Riishede M, Lassen AT, Baatrup G, Pietersen PI, Jacobsen N, Jeschke KN, Laursen CB. Point-of-care ultrasound of the heart and lungs in patients with respiratory failure: a pragmatic randomized controlled multicenter trial. Scand J Trauma Resusc Emerg Med. 2021;29(1):60.
doi pubmed - Ozkan B, Unluer EE, Akyol PY, Karagoz A, Bayata MS, Akoglu H, Oyar O, et al. Stethoscope versus point-of-care ultrasound in the differential diagnosis of dyspnea: a randomized trial. Eur J Emerg Med. 2015;22(6):440-443.
doi pubmed - Gundersen GH, Norekval TM, Haug HH, Skjetne K, Kleinau JO, Graven T, Dalen H. Adding point of care ultrasound to assess volume status in heart failure patients in a nurse-led outpatient clinic. A randomised study. Heart. 2016;102(1):29-34.
doi pubmed - Hacialiogullari F, Yilmaz F, Yilmaz A, Sonmez BM, Demir TA, Karadas MA, Duyan M, et al. Role of point-of-care lung and inferior vena cava ultrasound in clinical decisions for patients presenting to the emergency department with symptoms of acute decompensated heart failure. J Ultrasound Med. 2021;40(4):751-761.
doi pubmed - Araiza-Garaygordobil D, Gopar-Nieto R, Martinez-Amezcua P, Cabello-Lopez A, Manzur-Sandoval D, Garcia-Cruz E, De la Fuente-Mancera JC, et al. Point-of-care lung ultrasound predicts in-hospital mortality in acute heart failure. QJM. 2021;114(2):111-116.
doi pubmed - Via G, Hussain A, Wells M, Reardon R, ElBarbary M, Noble VE, Tsung JW, et al. International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr. 2014;27(7):683.e1-e33.
doi pubmed - Qaseem A, Etxeandia-Ikobaltzeta I, Mustafa RA, Kansagara D, Fitterman N, Wilt TJ, Clinical Guidelines Committee of the American College of P, et al. Appropriate use of point-of-care ultrasonography in patients with acute dyspnea in emergency department or inpatient settings: a clinical guideline from the American College of Physicians. Ann Intern Med. 2021;174(7):985-993.
doi pubmed - Sokolski M, Rydlewska A, Krakowiak B, Biegus J, Zymlinski R, Banasiak W, Jankowska EA, et al. Comparison of invasive and non-invasive measurements of haemodynamic parameters in patients with advanced heart failure. J Cardiovasc Med (Hagerstown). 2011;12(11):773-778.
doi pubmed - Greiner S, Jud A, Aurich M, Hess A, Hilbel T, Hardt S, Katus HA, et al. Reliability of noninvasive assessment of systolic pulmonary artery pressure by Doppler echocardiography compared to right heart catheterization: analysis in a large patient population. J Am Heart Assoc. 2014;3(4):e001103.
doi pubmed - Schwarz K, Ayyaz Ul Haq M, Sidhu B, Nolan J. Cardiac catheterization. Medicine. 2018;46(8):488-493.
doi - Ma TS, Paniagua D, Denktas AE, Jneid H, Kar B, Chan W, Bozkurt B. Usefulness of the sum of pulmonary capillary wedge pressure and right atrial pressure as a congestion index that prognosticates heart failure survival (from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness Trial). Am J Cardiol. 2016;118(6):854-859.
doi pubmed - Hajduczok AG, Muallem SN, Nudy MS, DeWaters AL, Boehmer JP. Remote monitoring for heart failure using implantable devices: a systematic review, meta-analysis, and meta-regression of randomized controlled trials. Heart Fail Rev. 2022;27(4):1281-1300.
doi pubmed - Adamson PB, Gold MR, Bennett T, Bourge RC, Stevenson LW, Trupp R, Stromberg K, et al. Continuous hemodynamic monitoring in patients with mild to moderate heart failure: results of The Reducing Decompensation Events Utilizing Intracardiac Pressures in Patients With Chronic Heart Failure (REDUCEhf) trial. Congest Heart Fail. 2011;17(5):248-254.
doi pubmed - Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB, CHAMPION Trial Study Group. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387(10017):453-461.
doi pubmed - Bourge RC, Abraham WT, Adamson PB, Aaron MF, Aranda JM, Jr., Magalski A, Zile MR, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol. 2008;51(11):1073-1079.
doi pubmed - Celik A, Surmeli A, Demir M, Esen K, Fural O, Camsari A. The early diagnostic value of chest X-ray scanning by the help of artificial intelligence in heart failure (Art-in-HF): the first outcomes. J Am Coll Cardiol. 2022;79(Suppl 9):395.
doi - Yasmin F, Shah SMI, Naeem A, Shujauddin SM, Jabeen A, Kazmi S, Siddiqui SA, et al. Artificial intelligence in the diagnosis and detection of heart failure: the past, present, and future. Rev Cardiovasc Med. 2021;22(4):1095-1113.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


