| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 13, Number 6, December 2022, pages 380-392
Anthracycline-Induced Cardiotoxicity in Breast Cancer Patients: A Five-Year Retrospective Study in 10 Centers
Ferdinand R. Gerodias Jra, b, m , Maria Katrina Tana, c, Arnold De Guzmand, Alisa Bernane, Sue Ann Locnena, c, f, Angela Apostol-Aldayg, Erwin Janino Ybanezf, Jose Donato Magnoh, Alvin Limi, Alex Juniaj, Ryan Mambulaok, Joanne Cosare-San Pedroa, Jonald Lucerol, Zaldy Quijanok, Josephine Apurillok, Arnold John Usonj, Jason Louie Limj, Christie Anne Insoj, Analigaya Agoncillo-Infantei, Roxanne Yen Bongcawilh, Gracieux Yuzon Fernandoh, Amanda Mae Ramos-Manalaysayh, Fe-Aileen Arellano-Simona, Elaine Marisse Ilagan-Cargulloa, Mariel Joy Bago-Azaresa, Jamil Baternaa, Julie Ann Tapispisani, Noelle Marie Masadao-Rodriguezi, Jannah Lee Tarranzah, Lorenz Sagayaga Listaf, Joar Kent Gumaponl
aSt. Luke’s Medical Center-QC, Quezon City, Philippines
bDepartment of Medicine, St. Luke’s Medical Center College of Medicine, William H. Quasha Memorial, Quezon City, Philippines
cSt. Luke’s Medical Center-GC, Bonifacio Global City, Philippines
dAngeles University Foundation Medical Center, Angeles City, Pampanga, Philippines
eDavao Doctors Hospital, Davao City, Davao del Sur, Philippines
fUniversity of the East Ramon Magsaysay Memorial Medical Center, Inc., Quezon City, Philippines
gCentral Luzon Doctors Hospital, Tarlac City, Tarlac, Philippines
hPhilippine General Hospital, Manila, Philippines
iUniversity of Santo Tomas (UST) Hospital, Manila, Philippines
jPerpetual Succour Hospital, Cebu City, Cebu, Philippines
kDona Remedios Trinidad Romualdez Hospital, Tacloban City, Leyte, Philippines
lVicente Sotto Memorial Medical Center, Cebu City, Cebu, Philippines
mCorresponding Author: Ferdinand R. Gerodias, Jr., Department of Medicine, St. Luke’s Medical Center College of Medicine, William H. Quasha Memorial, Quezon City, Philippines
Manuscript submitted October 13, 2022, accepted November 28, 2022, published online December 16, 2022
Short title: AIC in Filipino Breast Cancer Patients
doi: https://doi.org/10.14740/cr1442
| Abstract | ▴Top |
Background: Cardiotoxicity as a result of anthracycline chemotherapy has been linked to increased morbidity and mortality in breast cancer patients. There is a need for early detection through risk factor identification. To date, no large multicenter study has been conducted to describe the incidence, risk factors and clinical and demographic profiles of breast cancer patients with anthracycline-induced cardiotoxicity (AIC) in the Philippines.
Methods: This was a nationwide multicenter retrospective cohort study among adult breast cancer patients who underwent anthracycline chemotherapy from 2015 to 2020 in 10 sites in the Philippines. Baseline characteristics and possible risk factors for AIC were retrieved from medical records and cancer registries. AIC was defined as a reduction of left ventricular ejection fraction (LVEF) by > 10% from baseline to a value of < 53% or the development of overt left ventricular systolic dysfunction or heart failure (HF). Odds ratios from logistic regression were computed to determine risk factors associated with AIC using STATA-15.0 software.
Results: Out of 341 patients included, 33 had AIC, accounting for an incidence of 9.68%. Nine patients (2.6%) had clinical HF. AIC patients had a mean age of 53.91 ± 10.84 years. Breast cancer AIC patients were significantly older and had lower body mass index (BMI) than those without AIC. AIC patients had significantly more comorbidities, especially hypertension and atrial fibrillation. Multivariate analysis showed that patients with any preexisting comorbidity are approximately 12.37 times as likely to have AIC, while those with concurrent chemotherapy are 0.07 times or 93% less likely to have AIC.
Conclusion: Among adult breast cancer patients undergoing anthracycline chemotherapy, we determined a high incidence of cardiotoxicity at 9.68%. Having preexisting comorbidities gave patients 12 times increased odds of developing anthracycline cardiotoxicity. The presence of concurrent non-anthracycline chemotherapy showed an inverse association with the development of AIC which we attribute largely to patient selection in a retrospective study. The significantly higher propensity for AIC development in patients with preexisting comorbidities may warrant closer monitoring and control of patient comorbidities such as hypertension among patients undergoing anthracycline chemotherapy.
Keywords: Anthracyclines; Cardiomyopathy; Cardiotoxicity; Anthracycline-induced cardiotoxicity; Breast cancer
| Introduction | ▴Top |
Since their introduction in the 1960s, anthracyclines (doxorubicin, daunorubicin, epirubicin, and idarubicin) have been the chemotherapeutic drug class of choice for treating breast cancer [1], resulting in a dramatic improvement in overall survival rates [2]. Despite their proven efficacy, anthracyclines have their downside due to a myriad of adverse effects, the most serious of which is irreversible cardiotoxicity that may lead to clinical heart failure (HF).
The damage caused by the anthracyclines occurs in a cumulative dose-dependent fashion causing cardiomyocyte injury and death, leading to left ventricular (LV) dysfunction and HF [3]. Cardiac event rates on treatment were 7% at a cumulative anthracycline dose of 150 mg/m2, 18% at 350 mg/m2, and 65% at 550 mg/m2 [4]. In a review of over 43,000 patients with breast cancer, followed over a median of 53 months, anthracycline chemotherapy was associated with an adjusted hazard risk (HR) of 1.26 (confidence interval (CI): 1.12 - 1.42) for the development of HF in women aged 66 - 70 years [5].
The cardiotoxicity of anthracyclines may be acute, which is immediately after infusion; early, which occurs within the first year of treatment; or late, which manifests effects after several years. Acute toxicity develops in 1% of patients and is usually reversible. This includes transient LV dysfunction, supraventricular arrhythmias and electrocardiographic (ECG) changes. In patients who are > 65 years of age treated with commonly used anthracycline doses, the rate of anthracycline-associated HF can be as high as 10% [4]. Cardiotoxicity tends to occur more frequently in patients with the following factors: high cumulative anthracycline dose; extremes of age; female sex; preexisting conditions, such as cardiac diseases, diabetes mellitus (DM), hypertension (HTN), renal failure, and genetic factors; and with concurrent treatment with cyclophosphamide, trastuzumab, paclitaxel and prior mediastinal radiation therapy [6-9]. These risk factors predict patients who may be more prone to the development of cardiotoxicity, and thus would require more stringent surveillance.
Reductions of left ventricular ejection fraction (LVEF) provide an early warning of the development of cardiotoxicity even before overt symptomatic HF sets in. Echocardiography has been the method of choice to measure LVEF for screening and monitoring because of its wide availability, easy repeatability, versatility, lack of radiation exposure, and overall safety. A joint consensus statement by the American Society of Echocardiography and the European Society of Cardiovascular Imaging defined cancer therapeutics-related cardiac dysfunction (CTRCD) as a decrease in the LVEF of > 10 percentage points, to a value of < 53% [10]. Similarly, cardiotoxicity is defined by international oncologic guidelines as an absolute decrease in LVEF by more than 10% with a decline below the normal limit of 50% [11].
This paper describes the characteristics accompanying anthracycline-induced cardiotoxicity (AIC) in adult breast cancer patients in the Philippines. This information may provide reliable estimates of the clinical burden of AIC in the country. It may lead to increased awareness, index of suspicion, and surveillance of Filipino breast cancer patients with established risk factors for cardiotoxicity.
| Materials and Methods | ▴Top |
Study design and data sources
This was a retrospective cohort study done across 10 hospital sites in various locations in the Philippines. We reviewed the medical records of in- and outpatients, existing inventories, and registries of Cancer and Heart Institutes of these hospitals, and included data from all breast cancer patients who had anthracycline chemotherapy from June 2015 until June 2020. We recorded all transthoracic echocardiograms (TTEs) that were taken within 1 month before anthracycline chemotherapy as a baseline reading. Baseline demographics, clinical profile and presence or absence for prespecified risk factors for AIC were recorded at this time. We determined the occurrence of AIC from subsequent echocardiograms that were done 1 day up to 12 months post-chemotherapy. Patients were followed from the day of their initiation chemotherapy (index date) until 1 year after the completion of their chemotherapy. All chart data entries within the 1-year follow-up period of each included patient were reviewed for any recorded clinical outcome of symptomatic HF (Fig. 1).
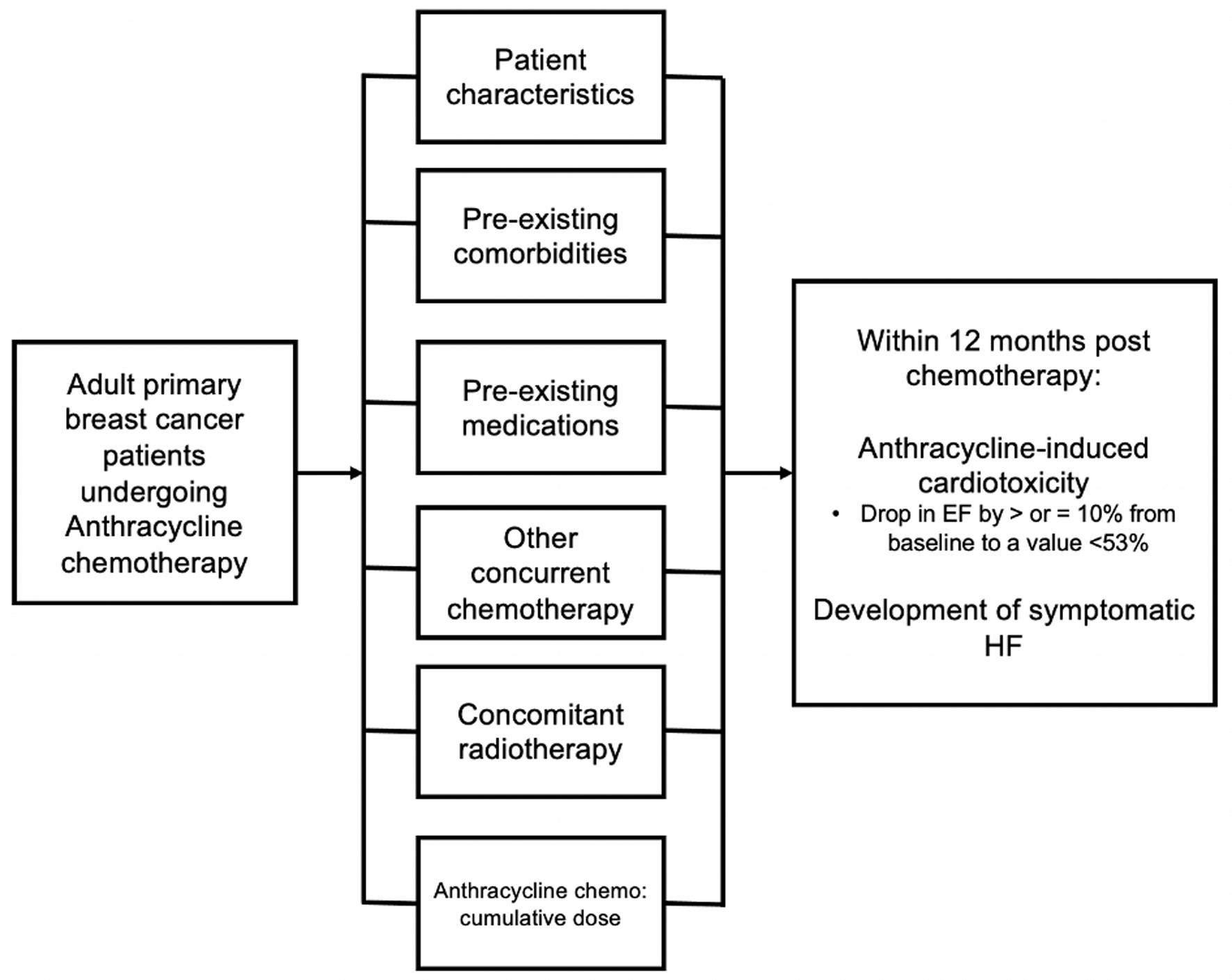 Click for large image | Figure 1. Conceptual framework for this study. |
Population
Inclusion and exclusion criteria
Patients who satisfied the following criteria were included: adult patients at least 19 years of age, with primary breast cancer, who underwent anthracycline chemotherapy (doxorubicin, epirubicin, or idarubicin) for the first time, regardless of concomitant chemoradiotherapy or number of cycles of anthracycline chemotherapy completed, with baseline TTE done within 1 month before initiation of anthracycline chemotherapy, and either of the following - TTE done 1 day up to 12 months after completing the course of chemotherapy, or TTE done during chemotherapy due to development of HF symptoms (i.e., orthopnea, exertional dyspnea, and paroxysmal nocturnal dyspnea).
The exclusion criteria were the following: patients with concomitant cancer (double primary), with debilitating disease or critical state disease, those with preexisting clinical HF or LV systolic dysfunction defined as LVEF < 52% in males and < 54% in females before chemotherapy, and patients who have received prior anthracycline chemotherapy.
Patients with preexisting clinical HF and LV systolic dysfunction were excluded because one of the main objectives of the study was to determine incident cases of HF that were mainly due to the administration of anthracyclines and not confounded by higher risk cardiovascular disease patients. Patients who had anthracyclines for the first time were included and those with prior anthracycline exposure were excluded, mainly to avoid duplicating patient entries, as cancer patients come in multiple times for their chemotherapy cycles. Patients were censored at 1 year from completion of chemotherapy as existing literature suggests that majority (98%) of AIC develop within the first year after chemotherapy had been completed [12]. A total of 341 patients were included in the final analysis.
Sample size calculation
A minimum of 340 breast cancer patients were required for this study based on a level of significance of 5%, assuming that 33.16% [13] of patients will develop cardiotoxicity, with the desired width of CI (precision) of 10% [14] as noted from the reference article by Sandamali et al, 2020 [13].
Research materials
Two-dimensional (2D) TTE results were obtained within 1 month before anthracycline chemotherapy initiation (baseline reading), and 1 day up to 12 months after completion of the anthracycline chemotherapy. Standard LVEFs were obtained. The LVEF measurements gathered were from the biplane Simpson’s method and/or Teichholz method, with or without contrast.
Additionally, the following pertinent data were obtained: demographics; preexisting comorbidities; preexisting medications; concurrent chemotherapeutic agents; radiation therapy; cumulative chemotherapeutic doses; all 2D TTE and dates (at baseline and post-chemotherapy up to 12 months); anthracycline chemotherapy (category of first session, doses, and dates of all anthracycline sessions; duration in months); date of the last follow-up; and if applicable, date and cause of death.
The main outcome considered is AIC, also referred to as CTRCD, as given by a categorical analysis of the reduction of LVEF by > 10% from baseline to a value of < 53% [10] or the development of clinical HF.
Ethical considerations
The study adhered to the ethical considerations and ethical principles set out in relevant guidelines, including the Declaration of Helsinki (2013), World Health Organization guidelines, International Conference on Harmonization-Good Clinical Practice, Data Privacy Act of 2012 of the Philippines, and the 2017 National Ethics Guidelines for Health and Health-related Research of the Philippines. The Clinical Protocol and all relevant documents were reviewed and approved by the Single Joint Research Ethics Board (SJREB), the Institutional Review Board of the Philippine Heart Association (PHA), and by the ethics review committees and Cancer registry keepers of the institutions included in the study. Patient confidentiality was respected by maintaining the anonymity of patient records. The investigators ensured the integrity of all data recorded.
Statistical analysis
Descriptive statistics were used to summarize the general and clinical characteristics of the participants. Frequency and proportion were used for categorical variables (nominal/ordinal), mean and standard deviation for normally distributed interval/ratio variables, and median and range for non-normally distributed interval/ratio variables. Independent sample t-test, Mann-Whitney U test, and Fisher’s exact/Chi-square test were used to determine the differences in mean, median, and frequency between those with AIC and those without, respectively. Crude odds ratios (ORs) and the corresponding 95% CIs from univariable and multivariable Firth logistic regression were computed to determine the association between patient characteristics and AIC. Variables significantly associated with AIC in the univariable models were included in the multivariable model. All valid data were included in the analysis. The null hypothesis was rejected at a 0.05 α-level of significance. STATA 15.0 software was used for data analysis.
| Results | ▴Top |
Patient demographics and clinical profile
A total of 3,579 breast cancer patients who underwent anthracycline chemotherapy were considered for inclusion in this study. Three thousand two hundred twenty-eight patients were excluded due to the following reasons: clinical ineligibility in 1,430 patients, incomplete or missing 2D echocardiograms in 1,568 patients, and date of initiation outside of study scope in 230 patients (Fig. 2).
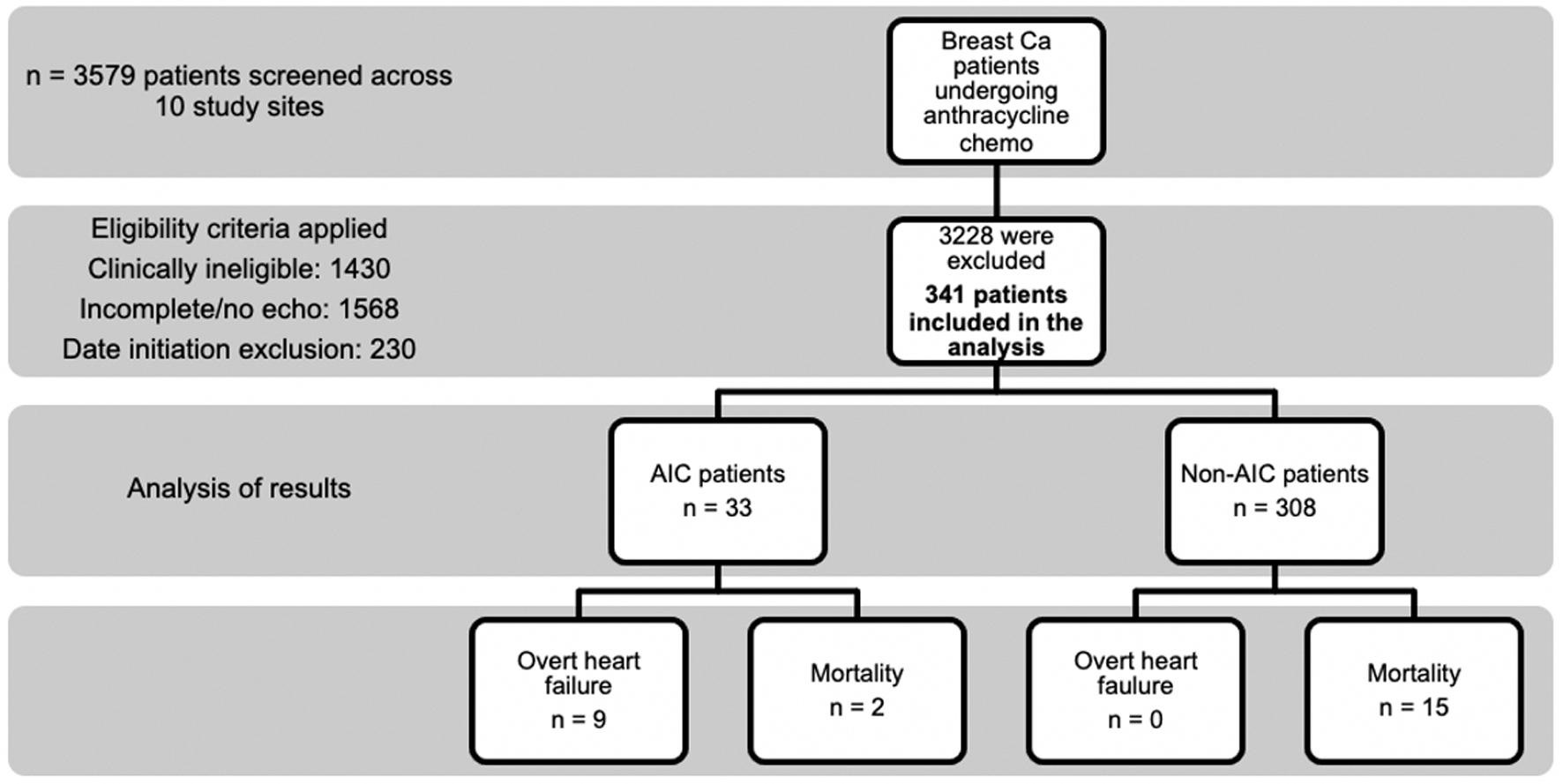 Click for large image | Figure 2. The study flow diagram of patient selection and clinical outcomes. |
Out of 341 patients reviewed in the final analysis, 338 (99.12%) were females (mean age was 53.91 ± 10.84 years) (Table 1). Patients with AIC were significantly older (59.06 ± 9.7 vs. 53.36 ± 10.83, P = 0.004) and had lower BMI (24 kg/m2 vs. 25.3 kg/m2, P = 0.003) than those without AIC. Moreover, 134 (39.3%) patients were overweight (BMI 23 - 27.5). Those who developed AIC had significantly more comorbidities (81.82% vs. 41.23%, P < 0.001). The most common comorbidities that we found in this cohort of patients were HTN (66.67%), DM (21.21%) and dyslipidemia (9.09%). Given their higher incidence of comorbidities, AIC patients also had more maintenance medications, such as angiotensin receptor blockers (ARBs, 13 or 39.39% vs. 40 or 12.99%, P ≤ 0.001), beta-blockers (6 or 18.18% vs. 12 or 4.22%, P = 0.006), and angiotensin converting enzyme (ACE) inhibitors (3 or 9.09% vs. 4 or 1.3%, P = 0.022).
 Click to view | Table 1. Demographic and Clinical Profile of Patients (n = 341) |
The AIC patients were less often on concurrent chemotherapeutic agents compared to those patients without AIC; namely, cyclophosphamide in only 36.36% of AIC patients vs. 81.17% in non-AIC patients (P ≤ 0.001), taxanes in 39.39% vs. 70.45% (P = 0.001) and 5-fluorouracil in 3.03% vs. 17.21% (P = 0.041). AIC patients had significantly lower mean baseline LVEF (65.89±5.84% vs. 67.71±4.89%, P = 0.047) (Table 1). In terms of chemotherapeutic management, more than half of the patients (54.55%) had chemotherapy on an outpatient basis (Table 2). AIC patients received a significantly lower median dose of epirubicin (14 mg/m2 vs. 70 mg/m2, P = 0.030).
 Click to view | Table 2. Chemotherapeutic Management (n = 341) |
Clinical outcomes
Thirty-three out of the 341 patients had AIC, accounting for an incidence of 9.68% (95% CI: 6.95 - 13.32); 2.6% of the total population had clinical HF (nine out of 341). There were a total of 17 deaths within the study period. The mortality rates were not statistically different between the AIC and non-AIC groups (P = 0.674). In the AIC group, the patients’ LVEF progressively decreased during the follow-up periods. Meanwhile, for patients in the non-AIC group, the LVEF values remained relatively constant from baseline to follow-up periods (Table 3).
 Click to view | Table 3. Clinical Outcomes (n = 341) |
Factors associated with AIC
The unadjusted OR showed that for every unit increase in age in years, the odds of AIC increased by approximately 5% (Table 4). Patients with comorbidities were six times more likely to have AIC compared to those without. Moreover, for those with HTN, the odds of AIC was five times higher, and for those with AF, it was 16 times higher. There was a significant increase in odds of AIC for patients who were on ARBs, beta-blockers, or ACE inhibitors. Patients treated with cyclophosphamide and taxanes were less likely to have AIC, 86.4% and 72.3% less likely, respectively. There was an increased odds by 28 times among those given vinorelbine.
 Click to view | Table 4. Unadjusted Odds Ratio for Factors Associated With Anthracycline-Induced Cardiotoxicity (n = 341) |
Table 5 shows the multivariate analysis of selected independent variables. The presence of comorbidities and concurrent non-anthracycline chemotherapy were confirmed as independent correlates of AIC but not age, medications, baseline LVEF, doxorubicin dosage > 250 mg/m2 and epirubicin dosage > 600 mg/m2. Patients with any comorbidity were approximately 12.37 times more likely to have AIC, while those with concurrent chemotherapy were 0.07 times or 93% less likely to have AIC.
 Click to view | Table 5. Multivariate Analysis for Factors Associated With Anthracycline-Induced Cardiotoxicity (n = 341) |
| Discussion | ▴Top |
This is a retrospective study of a cohort of breast cancer patients who underwent anthracycline chemotherapy and who had no preexisting cardiac dysfunction from 10 centers in various locations in the Philippines. We report on the incidence of, and the risk factors associated with, AIC.
Incidence of AIC and HF
The foremost finding of this study is that AIC occurred at a frequency of 9.68% (33 cases in 341 patients) in this cohort of patients in the Philippines. This is lower compared to the results from other studies done in Asia where the incidence ranged from 25% to 33%. This may in part be due to the use of varying definitions of AIC [13, 15, 16]. In this study, the investigators strictly followed the definition of the American and European Society of Echocardiography Expert Consensus of Anthracycline-induced cardiotoxicity, which is a reduction of LVEF by more than 10%, to a value below 53% [10].
It is of concern that a significant proportion of patients with AIC had developed symptomatic HF (nine out of 33, accounting to 27%). A study by Cardinale et al [17] showed that early intervention with traditional medications after the detection of HF may potentially reverse LV systolic dysfunction. They reported a response rate of 64% when the HF regimen was given < 2 months, as compared to only 7% when given 4 - 6 months after detection of AIC [17]. This emphasizes the importance of cardiac monitoring and early detection for cardiotoxicity in these sets of patients. In our study, we have detected high rates of breast cancer patients not being screened and/or being followed with cardiac imaging as reflected in the high exclusion rates (Fig. 2). It is therefore possible that the high proportion of patients developing HF may be a reflection of the strength of the current local practice. Our country, the Philippines, is just starting to gain experience in the field of cardio-oncology in the past decade. The authors advocate emphasis on clinician education and cardiac monitoring in the community to improve the cardiac safety of anthracycline chemotherapy.
Risk factors associated with the development of AIC
Previous studies have determined the risk factors for AIC. These risk factors included cumulative anthracycline doses, > 65 years of age, baseline LVEF, renal failure, concomitant or previous radiation therapy involving the heart, concurrent chemotherapy (alkylating or antimicrotubule agents, immune- and targeted-therapies), and preexisting conditions such as cardiac diseases, HTN, and genetic factors [4-6].
Multivariate analysis showed that having comorbidities gave patients 12 times increased odds of developing anthracycline cardiotoxicity. In the cohort, patients with AIC had a high prevalence of comorbidities, particularly HTN at 66.67%, DM at 21.21%, and dyslipidemia at 9.09%. The possible mechanism for potentiation for risk of AIC among patients with HTN is related to oxidative stress and inflammatory processes. Anthracyclines can trigger abnormal cell signaling and cytotoxic molecules, which are potentiated by the same abnormal cell signaling as with that of HTN [18]. A meta-analysis by Qui et al among 7,488 patients receiving anthracycline chemotherapy showed that HTN had an OR of 1.99 (95% CI: 1.43 - 2.76) in the development of AIC. Likewise, DM was also found to have increased odds of AIC (OR: 1.74; 95% CI: 1.11 - 2.74) [19]. Mechanistically, DM potentiates anthracycline cardiotoxicity by exposing the heart to high blood sugar, thereby increasing fatty acids and cytokines. This then leads to continuous accumulation of fat droplets in myocardial cells and eventually cardiotoxicity [20]. Lastly, dyslipidemia was also shown to be related to increased risk for AIC as reported in other studies [12, 21]. Its proposed mechanism in potentiating AIC involves endothelial dysfunction and vascular complications [22]. The significantly higher propensity for AIC development in patients with comorbidities may warrant closer monitoring and control of HTN, DM, and dyslipidemia before and during the chemotherapeutic sessions.
This retrospective study included breast cancer patients who were given different anti-cancer therapies, such as cyclophosphamide, taxanes, and human epidermal growth factor 2 (Her-2) inhibitors, whose mechanism for cardiotoxicity differs from that of anthracyclines. In many cases, these non-anthracycline chemotherapies, like Her-2 inhibitors, had reversible cardiotoxic effects and thus patients may spontaneously recover [23]. This is in contrast to the irreversible cardiotoxic effect of anthracyclines.
Findings of this study showed that patients who were concurrently given non-anthracycline chemotherapy had a significantly less likelihood of cardiotoxicity than those given anthracyclines alone. On the other hand, existing literature proposes that having concurrent chemotherapy apart from anthracyclines predisposes patients to AIC [7]. An explanation for our contrasting findings is that the temporality of EF determination could have had a significant confounding effect (i.e., those who had cardiotoxicity from other chemotherapy agents could have already recovered from the damage at the time of EF determination, and thus, the cardiotoxicity was not detected). Additionally, we found that those patients with concurrent non-anthracycline chemotherapies had a lower mean dose of doxorubicin of 155.77 mg/m2 compared to those patients who were given only doxorubicin regimens (higher mean dose of 172.69 mg/m2). An accepted fact is that oncologists will classify and determine which patients will receive concurrent chemotherapy based on their baseline clinical profile. Oncologists tend to give concurrent chemotherapy to younger patients and to those with less or no comorbidities. Hence, these patients possibly receive a lower total cumulative dose of anthracyclines. Older patients as well as those with comorbidities who are poor candidates for concurrent therapy, tend to receive anthracyclines alone or in sequential therapy with varying cumulative anthracycline doses [24]. In our study, the mean age of patients who had concurrent chemotherapy was lower at 53.66 compared to 57.45 years for those who had anthracyclines alone. Likewise, patients who received concurrent chemotherapy had no comorbidity in 56% of patients, compared to only 36% among patients who had anthracyclines alone. A prospective, randomized and controlled study involving subsets of patients with more comorbidities and with a high risk of AIC may be needed to verify our findings.
In this study, the mean age was only 53.91 years, with those over 65 years comprising only 12.31% of the total cohort. The inclusion of a relatively younger cohort of patients may preclude a definitive assessment that having an age over 65 is a significant risk factor for the development of AIC. Likewise, we did not demonstrate an independent correlate of cumulative anthracycline dose for AIC development. However, the cumulative doxorubicin dose in the cohort was relatively low, with a median dose of only 132 mg/m2 in the AIC group. This is in contrast to the reported higher risk for AIC development for doxorubicin dose going beyond 250 mg/m2 [8, 9, 12]. It is, however, noteworthy, that we still detected a high incidence of AIC despite having a lower mean age and a lower mean anthracycline dose. These results are similar to that of a retrospective study done on 99 breast cancer Filipino female patients, wherein the mean age of those who developed CTRCD was relatively young as well (56 years), with an incidence for subclinical LV dysfunction of 18% [25]. Similar to our study, patients in their cohort also received relatively lower doses of anthracyclines. Therefore, clinicians should also be mindful of the possibility of AIC even for younger patients or those with lower anthracycline doses.
To the best of our knowledge, this study is the first multi-site real world data in a Filipino cohort to determine the national burden of AIC. We believe that this is an important step in improving our knowledge and care for cancer patients undergoing anthracycline chemotherapy. Our findings show that Filipino breast cancer patients may have lower thresholds or propensities for AIC in terms of age and cumulative anthracycline dose compared to other ethnicities. We believe that this is worth investigating further in future studies.
Limitations of the study
The measurement accuracy of the LVEF is dependent on the image quality and expertise of the sonographer and reader. Therefore, the determination of the EF was subjected to some degree of inter-vendor and inter-observer variability. Some AIC may have been potentially undetected, leading to an underestimation of its incidence. Moreover, the duration of patient follow-up was limited to only 1 year from the completion of anthracycline chemotherapy and it was, therefore, possible to miss out on those patients who developed AIC beyond 1 year. Given the retrospective nature of the study, a sizable number of patients were excluded (1,568 patients) mainly due to lack of matching pre- and/or post-chemotherapy echocardiograms. Despite current recommendations to perform a TTE prior to anthracycline initiation as well as periodic LVEF monitoring [26], many patients did not have either one or both. This reduced the generalizability of our results, and may have led to an underestimation of the true incidence of AIC.
Patients with preexisting cardiac dysfunction were excluded in this study. This allowed the assessment of the patient’s risk for the development of cardiotoxicity from anthracyclines alone, without the confounding etiologies of LV dysfunction such as ischemic heart disease and valvular heart diseases. It is worth mentioning, however, that other important potential preexisting cardiac conditions not routinely seen in medical chart review may be missed in our study. Hypertrophic cardiomyopathy (HCM) [27], arrhythmogenic right ventricular cardiomyopathy [28], dilated cardiomyopathy [29] and cardiomyopathies induced by arrhythmias [30] may have normal LVEF on TTE and yet can still present with overt heart failure and even sudden cardiac death later in their disease. Moreso, these patients may present with symptoms not typical of HF, i.e., patients with HCM can present with syncope [27]. A comprehensive account of all these cardiac disease entities at baseline through symptom screening may be done in future studies. Data on the amount of radiation the patients had received were not available for analysis, limiting our ability to accurately assess its association with AIC. Lastly, our study did not stratify based on breast cancer staging. Higher stages may necessitate more cycles of chemotherapy, hence longer lines of treatment and more prolonged exposure to anthracyclines and other potentially cardiotoxic therapies. Lower stage breast cancers in contrast may entail lower dose exposure of anthracyclines.
Conclusion
In a 5-year nationwide multicenter cohort study in the Philippines, we determined a high incidence of cardiotoxicity at 9.68% among adult breast cancer patients undergoing anthracycline chemotherapy. Concurrent non-anthracycline chemotherapy showed an inverse association with the development of AIC. We attribute this inverse association to patient selection, as the patients who had concurrent chemotherapy had received lower mean anthracycline doses and had fewer or no preexisting comorbidities. Having preexisting comorbidities increased the odds of developing anthracycline cardiotoxicity. This may warrant closer monitoring and control of patient comorbidities such as HTN among patients undergoing anthracycline chemotherapy.
Acknowledgments
This study is a project of the PHA CV-Oncology council. We would like to thank the 2021 Philippine Heart Association Board for granting us full funding and support in the execution of the study. Our sincere thanks also to the entire ANICA research team (including our research assistants and coordinators); the success of this research would not have been possible without their tireless efforts.
Financial Disclosure
The authors report no disclosures. This study was fully funded by the Philippine Heart Association.
Conflict of Interest
The authors declare no potential conflict of interest.
Informed Consent
This study was an observational retrospective cohort study using chart review of medical records as the method of data collection. There was no actual interaction with patients, thus, the Single Joint Research Ethics Board of the Philippines granted the study a waiver for informed consent.
Author Contributions
Study conception and design: Ferdinand Jr. Gerodias, Maria Katrina Tan, Arnold De Guzman, Alisa Bernan, Sue Ann Locnen. Data collection: Maria Katrina Tan, Alisa Bernan, Sue Ann Locnen, Angela Apostol-Alday, Erwin Janino Ybanez, Jose Donato Magno, Alvin Lim, Alex Junia, Ryan Mambulao, Joanne Cosare-San Pedro, Jonald Lucero, Zaldy Quijano, Josephine Apurillo, Arnold John Uson, Jason Louie Lim, Christie Anne Inso, Analigaya Agoncillo-Infante, Roxanne Yen Bongcawil, Gracieux Yuzon Fernando, Amanda Mae Ramos-Manalaysay, Fe-Aileen Arellano-Simon, Elaine Marisse Ilagan-Cargullo, Mariel Joy Bago-Azares, Jamil Baterna, Julie Ann Tapispisan, Noelle Marie Masadao-Rodriguez, Jannah Lee Tarranza, Lorenz Sagayaga Lista, Joar Kent Gumapon. Analysis and interpretation of results: Ferdinand Jr. Gerodias, Maria Katrina Tan, Arnold De Guzman, Alisa Bernan, Sue Ann Locnen, Angela Apostol-Alday, Erwin Janino Ybanez, Jose Donato Magno, Alvin Lim, Alex Junia, Ryan Mambulao, Joanne Cosare-San Pedro, Jonald Lucero. Draft manuscript preparation: Ferdinand Jr. Gerodias, Maria Katrina Tan, Arnold De Guzman. All authors reviewed the results and approved the final version of the manuscript. All authors agree to be accountable for all aspects of the work including the accuracy and integrity of the results of the study.
Data Availability
The data that support the findings of this study are available from the corresponding author, FRG, upon request of the editor/s and/or reviewer/s.
Abbreviations
2D: two-dimensional; ACE: angiotensin converting enzyme; AF: atrial fibrillation; AIC: anthracycline-induced cardiotoxicity; ARBs: angiotensin receptor blockers; BMI: body mass index; CAD: coronary artery disease; CTRCD: cancer therapeutics-related cardiac dysfunction; DM: diabetes mellitus; ECG: electrocardiogram; HER2: human epidermal growth factor 2; HF: heart failure; HR: hazard risk; LVEF: left ventricular ejection fraction; PHA: Philippine Heart Association; SJREB: Single Joint Research Ethics Board; TTE: transthoracic echocardiogram
| References | ▴Top |
- Bansal N, Adams MJ, Ganatra S, Colan SD, Aggarwal S, Steiner R, Amdani S, et al. Strategies to prevent anthracycline-induced cardiotoxicity in cancer survivors. Cardiooncology. 2019;5:18.
doi pubmed - Hershman DL, Shao T. Anthracycline cardiotoxicity after breast cancer treatment. Oncology (Williston Park). 2009;23(3):227-234.
- Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart. 2018;104(12):971-977.
doi pubmed - Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869-2879.
doi pubmed - Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25(25):3808-3815.
doi pubmed - Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clin Proc. 2014;89(9):1287-1306.
doi pubmed - Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768-2801.
doi pubmed - Volkova M, Russell R, 3rd. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr Cardiol Rev. 2011;7(4):214-220.
doi pubmed - Fradley MG, Neilan TG. Cardiovascular outcomes in anthracycline-related cardiomyopathy: when a p value of >0.05 is a good outcome! JACC Clin Electrophysiol. 2017;3(2):151-153.
doi pubmed - Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911-939.
doi pubmed - Rosenthal DS, Braunwald E. Cardiac effects of radiation therapy and chemotherapy. In: Braunwald E, ed. Heart disease: a textbook of cardiovascular medicine. Philadelphia, Pa: WB Saunders Co. 2001:1746-1751.
- Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981-1988.
doi pubmed - Sandamali JAN, Hewawasam RP, Fernando M, Jayatilaka K, Madurawe RD, Sathananthan PP, Ekanayake U, et al. Anthracycline-induced cardiotoxicity in breast cancer patients from Southern Sri Lanka: an echocardiographic analysis. Biomed Res Int. 2020;2020:1847159.
doi pubmed - Peacock JL, Peacock PJ. Research design. Oxford Handbook of Medical Statistics. United States: Oxford University Press; 2011:60-61.
doi - Shaikh AS, Saleem AF, Mohsin SS, Alam MM, Ahmed MA. Anthracycline-induced cardiotoxicity: prospective cohort study from Pakistan. BMJ Open. 2013;3(11):e003663.
doi pubmed - Khattry N, Malhotra P, Grover A, Sharma SC, Varma S. Doxorubicin-induced cardiotoxicity in adult Indian patients on chemotherapy. Indian J Med Paediatr Oncol. 2009;30(1):9-13.
doi pubmed - Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, Rubino M, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55(3):213-220.
doi pubmed - Kuriakose RK, Kukreja RC, Xi L. Potential therapeutic strategies for hypertension-exacerbated cardiotoxicity of anticancer drugs. Oxid Med Cell Longev. 2016;2016:8139861.
doi pubmed - Qiu S, Zhou T, Qiu B, Zhang Y, Zhou Y, Yu H, Zhang J, et al. Risk factors for anthracycline-induced cardiotoxicity. Front Cardiovasc Med. 2021;8:736854.
doi pubmed - Valcovici M, Andrica F, Serban C, Dragan S. Cardiotoxicity of anthracycline therapy: current perspectives. Arch Med Sci. 2016;12(2):428-435.
doi pubmed - Piotrowski G, Gawor R, Stasiak A, Gawor Z, Potemski P, Banach M. Cardiac complications associated with trastuzumab in the setting of adjuvant chemotherapy for breast cancer overexpressing human epidermal growth factor receptor type 2 - a prospective study. Arch Med Sci. 2012;8(2):227-235.
doi pubmed - Pi X, Xie L, Patterson C. Emerging Roles of Vascular Endothelium in Metabolic Homeostasis. Circ Res. 2018;123(4):477-494.
doi pubmed - Mohan N, Jiang J, Dokmanovic M, Wu WJ. Trastuzumab-mediated cardiotoxicity: current understanding, challenges, and frontiers. Antib Ther. 2018;1(1):13-17.
doi pubmed - Guo F, Yi Z, Wang W, Han Y, Yu P, Zhang S, Ouyang Q, et al. Profile, treatment patterns, and influencing factors of anthracycline use in breast cancer patients in China: A nation-wide multicenter study. Cancer Med. 2021;10(19):6744-6761.
doi pubmed - Manaloto JGG, Cruz-Tan MK, Tiongco RH, Jimenez RM, Cornelio GH, et al. Clinical outcomes of chemotherapy-induced subclinical left ventricular systolic dysfunction among breast cancer patients detected using echocardiographic global longitudinal strain. Eur Heart J. 2020;41(Suppl 2):ehaa946.3264.
doi - Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, Herrmann J, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31(2):171-190.
doi pubmed - Mascia G, Crotti L, Groppelli A, Canepa M, Merlo AC, Benenati S, Di Donna P, et al. Syncope in hypertrophic cardiomyopathy (part I): An updated systematic review and meta-analysis. Int J Cardiol. 2022;357:88-94.
doi pubmed - Carrick RT, Te Riele A, Gasperetti A, Bosman L, Muller SA, Pendleton C, Tichnell C, et al. Longitudinal prediction of ventricular arrhythmic risk in patients with arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2022;15(11):e011207.
doi pubmed - Shams P, Sultan FAT. Clinical characteristics, cardiac magnetic resonance features, and outcomes of patients with dilated cardiomyopathy - an experience from a South Asian country. J Clin Imaging Sci. 2021;11:40.
doi pubmed - Sun Y, Yu X, Xiao X, Dai S, Zhang R, Wang Z, Ma C, et al. Cardiomyopathy induced by premature ventricular contractions with ventricular escape beats in the compensatory pause: A case report and brief review of the literature. Medicine (Baltimore). 2022;101(34):e30277.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


