| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 14, Number 3, June 2023, pages 183-191
Obstructive Sleep Apnea as a Predictor of Inducible Atrial Flutter During Pulmonary Vein Isolation in Patients With Atrial Fibrillation: Clinical Significance and Follow-Up Outcomes
John Taylora, d, Sohiub N. Assafa, Abdallah N. Assafa, Eric Heidelc, William Mahlowa, b, Raj Baljepallya, b
aThe University of Tennessee Graduate School of Medicine, Knoxville, TN 37920, USA
bDivision of Cardiology, University of Tennessee Medical Center Knoxville, Knoxville, TN 37920, USA
cDepartment of Surgery, University of Tennesse, Knoxville, TN 37920, USA
dCorresponding Author: John Taylor, The University of Tennessee Graduate School of Medicine, Knoxville, TN 37920, USA
Manuscript submitted March 9, 2023, accepted May 1, 2023, published online May 26, 2023
Short title: OSA Predicts Inducible AFL During PVI
doi: https://doi.org/10.14740/cr1491
| Abstract | ▴Top |
Background: Atrial fibrillation (AF) and atrial flutter (AFL) often coexist in patients and may lead to severe symptoms and complications. Despite their coexistence, prophylactic cavotricuspid isthmus (CTI) ablation has failed to reduce the incidence of recurrent AF or new onset AFL. In contrast, the presence of inducible AFL during pulmonary vein isolation (PVI) has been shown to be predictive of symptomatic AFL during follow-up. However, the potential role of obstructive sleep apnea (OSA) as a predictor of inducible AFL during PVI in patients with AF remains unclear. Therefore, this study aimed to examine the potential role of OSA as a predictor of inducible AFL during PVI in patients with AF and reexamine the clinical significance of inducible AFL during PVI in terms of recurrent AFL or AF during follow-up.
Methods: We conducted a single-center, non-randomized retrospective study on patients who underwent PVI between October 2013 and December 2020. A total of 192 patients were included in the study after screening 257 patients for exclusion criteria, which included a previous history of AFL or previous PVI or Maze procedure. All patients underwent a transesophageal echocardiogram (TEE) prior to their ablation to rule out a left atrial appendage thrombus. The PVI was performed using both fluoroscopic and electroanatomic mapping derived from intracardiac echocardiography. After the confirmation of PVI, additional electrophysiology (EP) testing was performed. AFL was classified as typical or atypical based on the origin and activation pattern. Descriptive and frequency statistics were performed to describe the demographic and clinical characteristics of the sample, and Chi-square and Fisher’s exact tests were used to compare independent groups on categorical outcomes. Logistic regression analysis was performed to adjust for confounding variables. The study was approved by the Institutional Review Board, and informed consent was waived given the retrospective nature of the study.
Results: Of the 192 patients included in the study, 52% (n = 100) had inducible AFL after PVI, with 43% (n = 82) having typical right AFL. Bivariate analysis showed statistically significant differences between the groups for OSA (P = 0.04) and persistent AF (P = 0.047) when examining the outcome of any inducible AFL. Similarly, only OSA (P = 0.04) and persistent AF (P = 0.043) were significant when examining the outcome of typical right AFL. Multivariate analysis showed that only OSA was significantly associated with any inducible AFL after controlling for other variables (adjusted odds ratio (AOR) = 1.92, 95% confidence interval (CI): 1.003 - 3.69, P = 0.049). Of the 100 patients with inducible AFL, 89 underwent additional ablation for AFL prior to completion of their procedure. At 1 year, the rates of recurrence for AF, AFL, and either AF or AFL were 31%, 10%, and 38%, respectively. There was no significant difference in the rates of recurrence of AF, AFL, or either AF/AFL at 1 year when accounting for the presence of inducible AFL or the efficacy of additional AFL ablation.
Conclusions: In conclusion, our study found a high incidence of inducible AFL during PVI, particularly among patients with OSA. However, the clinical significance of inducible AFL in relation to the recurrence rates of AF or AFL at 1-year post-PVI remains unclear. Our findings suggest that successful ablation of inducible AFL during PVI may not provide clinical benefit in reducing AF or AFL recurrence. To establish the clinical significance of inducible AFL during PVI in various patient populations, further prospective studies with larger sample sizes and longer follow-up periods are necessary.
Keywords: Pulmonary vein isolation; Inducible atrial flutter; Atrial fibrillation ablation; Repeat catheter ablation; Pulmonary vein reconnection; Atrial flutter
| Introduction | ▴Top |
Atrial fibrillation (AF) and atrial flutter (AFL) often coexist in patients, and it is not uncommon for a person with AF to also experience episodes of AFL. In fact, studies have shown that the presence of AF may increase the likelihood of developing AFL, and vice versa [1, 2]. One study conducted by the Euro Heart Survey found that approximately 25% of patients with AF also had AFL [1]. Another registry study of 407 consecutive patients found that AF and AFL were present together in about 15% of cases [2].
Furthermore, the coexistence of AF and AFL may be associated with more severe symptoms and complications. For example, a nationwide cohort study in Taiwan found that patients with both AF and AFL had a significantly higher risk of adverse outcomes, such as stroke and heart failure, compared to patients with either AF or AFL alone [3]. These findings are consistent with other studies that have suggested a link between the coexistence of AF and AFL and increased morbidity and mortality [1, 2].
Despite the coexistence of AF and AFL, several studies have shown that prophylactic cavotricuspid isthmus (CTI) ablation failed to reduce the incidence of recurrent AF or new onset AFL and did not increase AF-free survival time [4-6]. For instance, the randomized control trial by Pontoppidan et al showed that prophylactic CTI ablation failed to reduce the incidence of recurrent AF or new onset AFL [4]. A second randomized control trial by Kim et al showed similar results with no evidence of reduced incidence of recurrent AF or new onset AFL [5]. A cohort study with a large number of patients by Mesquita et al also showed that prophylactic CTI ablation failed to increase AF-free survival time [6].
On the contrary, the presence of inducible flutter during pulmonary vein isolation (PVI) has been shown to be predictive of symptomatic AFL during follow-up. To date, only one study has investigated the clinical significance of inducible AFL during PVI in patients with AF. In this study, a history of AFL was found to be the only variable associated with inducibility of AFL during PVI. Notably, this study did not examine the potential role of obstructive sleep apnea (OSA) as a predictor. The same study found that AFL occurs in at least one-third of patients with AF and may be observed in two-thirds of patients undergoing PVI for AF. Additionally, typical AFL was observed in about 80% of these episodes. The occurrence of typical AFL during a PVI procedure was predictive of symptomatic AFL during follow-up after PVI. These findings suggested that it may be appropriate to ablate the CTI whenever typical AFL is observed in the course of a catheter ablation procedure aimed at the elimination of AF [7].
Given the known relationship between OSA and atrial arrhythmias, the aim of this study was twofold. First, we sought to examine the potential role of OSA as a predictor of inducible AFL during PVI in patients with AF. Second, we sought to reexamine whether the presence of inducible AFL during PVI had clinical significance in terms of recurrent AFL or AF during follow-up.
| Materials and Methods | ▴Top |
Study design
This was a single-center non-randomized retrospective study performed on patients requiring PVI between October 2013 and December 2020. A total of 257 that underwent PVI were initially screened for our exclusionary criteria, which included a previous history of AFL, or history of previous PVI or Maze procedures.
Study population
The study included patients between October 2013 and December 2020 that required PVI as clinically indicated by the presence of persistent or treatment resistant paroxysmal AF. A total of 257 that underwent PVI were initially screened for our exclusionary criteria, which included a previous history of AFL or previous PVI or Maze procedure. A total remaining cohort of 192 patients were included in the study. All procedures were conducted with informed consent. All patients underwent a transesophageal echocardiogram (TEE) prior to their ablation to rule out a left atrial appendage thrombus. All patients underwent PVI through a femoral vein approach using both fluoroscopic and electroanatomic mapping derived from intracardiac echocardiography. Following confirmation of PVI, patients underwent additional electrophysiology (EP) testing using a deflectable decapolar electrophysiological catheter that was positioned in the coronary sinus. Decremental pacing was performed until the atrioventricular (AV) node effective refractory period (ERP) was reached, after which burst atrial pacing with atrial extra stimuli was deployed in attempts to induce atrial dysrhythmias (Fig. 1). The procedures were conducted using standard EP laboratory equipment and techniques.
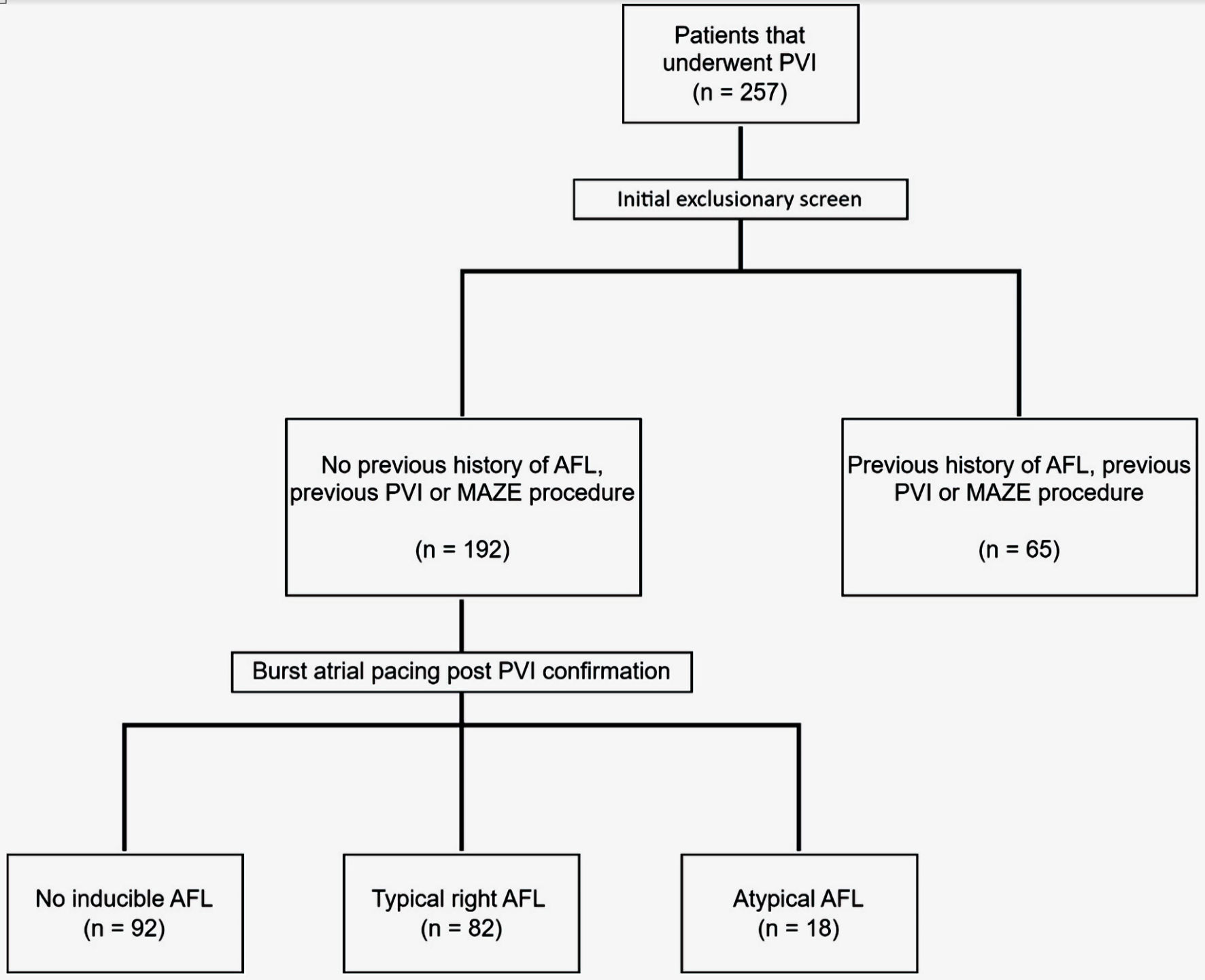 Click for large image | Figure 1. Flow diagram illustrating patient selection. AFL: atrial flutter; PVI: pulmonary vein isolation; Maze: Cox maze procedure. |
AFL was classified as “typical” if it was determined to be arising from the right atrium and involving macro reentry through the CTI, as evidenced by the presence of a counterclockwise or clockwise activation on electromagnetic mapping. When electroanatomic mapping was not used, the presence of a right to left concentric activation pattern on the coronary sinus catheter was used to infer the origin of the AFL in the right atrium. AFL was considered “atypical” if it was found to be arising from the left atrium, or if the AFL was non-sustained, or if it did not exhibit the typical activation pattern that would suggest involvement of the CTI.
Statistical analysis
A priori sample size calculation was performed for the purpose of collecting a large enough sample to have adequate statistical power. The researchers hypothesized a proportion of OSA in the “any inducible AFL” group of 60% and a proportion of 40% for OSA in the control group. Using a two-tailed hypothesis, the aforementioned proportions for 60% versus 40%, an alpha value of 0.05, and beta value of 0.20 (1-beta = 0.80; 80%), and equal allocation to treatment arms (1:1), a total of 97 participants would be needed in each treatment arm for a total sample size of 192.
Descriptive and frequency statistics were performed to describe the demographic and clinical characteristics of the sample. Chi-square and Fisher’s exact tests were performed to compare independent groups on categorical outcomes. Frequencies and percentages were reported and interpreted for the analyses of categorical outcomes. The statistical assumption of normality was assessed using Shapiro-Wilk test and when the assumption was violated, non-parametric Mann-Whitney U tests were used to compare the groups on continuous outcomes. Medians and interquartile ranges were reported for the comparisons of continuous outcomes. Logistic regression analysis was performed to adjust for confounding, demographic, and clinical variables when predicting for a binary, categorical outcome. Adjusted odds ratios (AOR) with 95% confidence interval (95% CI) were reported for the logistic regression analyses. Statistical significance was assumed at an alpha value of 0.05 and all analyses were performed using SPSS version 29 (Armonk, NY: IBM Corp.). Additional statistical and graphical analysis were performed using GraphPad Prism version 9.5.1 (Boston, MA: Dotmatics LLC.).
Ethical considerations
The study was approved by the Institutional Review Board (IRB) at the University of Tennessee Graduate School of Medicine, Knoxville, TN with IRB number 4326. Informed consent was waived by the IRB, given the retrospective nature of the study. The study was conducted in compliance with the institution’s ethical standards and the revised Declaration of Helsinki.
| Results | ▴Top |
Baseline characteristics
A total of 192 patients were included in the study after exclusion of patients with history of PVI, Maze, AFL, or CTI ablation (n = 65). The median age was 66 (interquartile range (IQR): 49 - 71) and the median body mass index (BMI) was 32 (IQR: 28 - 37). Of the 192 patients, 37% of the patients were female and 53% had persistent AF. Table 1 shows the baseline patient demographics. Table 2 shows the baseline background medical therapy.
 Click to view | Table 1. Baseline Characteristics of the Patients |
 Click to view | Table 2. Patient Home Medications and Antiarrhythmic Therapy |
Preoperative TEE
All patients received a TEE the day of their procedure to exclude left atrial appendage thrombus. The median ejection fraction (EF) was 55%. Table 3 shows the baseline echocardiogram parameters.
 Click to view | Table 3. Transesophageal Echocardiogram Prior to PVI |
AFL induction
After completion of the PVI, patients underwent rapid atrial pacing in attempts to induce AFL. AFL was induced in 52% of the patients (n = 100). Typical right AFL occurred in 43% of patients (n = 82). Figure 2 shows the incidence of inducible AFL. AFL ablation was performed on 89 of the 100 patients with inducible AFL after completion of the PVI.
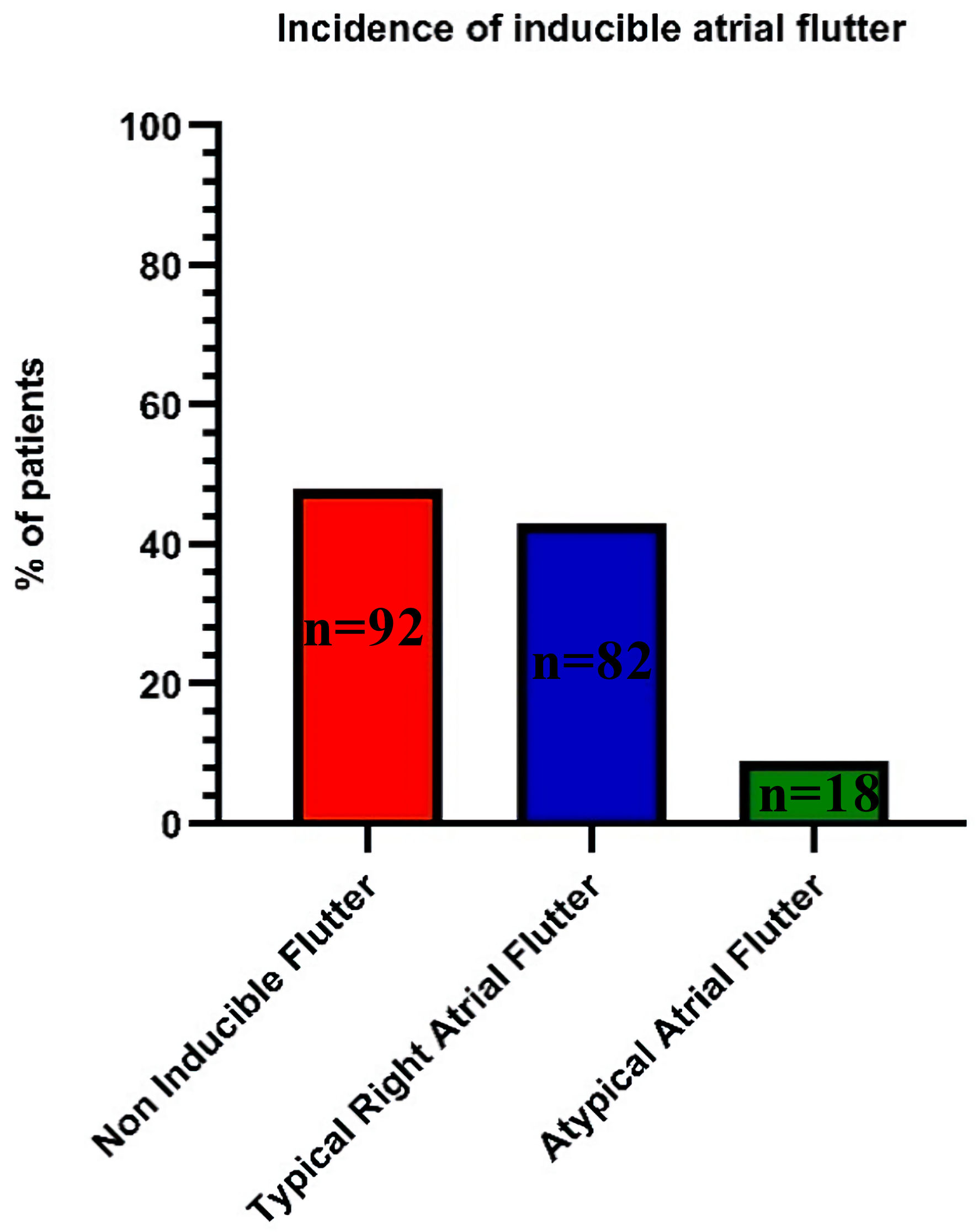 Click for large image | Figure 2. Primary outcome inducible atrial flutter. |
Bivariate analysis
Bivariate analysis was performed on the patients’ baseline demographics and preop TEE findings to determine risk factors for inducible AFL at the time of PVI. Categorial variables were examined via Chi-square analysis for the outcomes of any inducible AFL, typical right AFL, and atypical AFL. When comparing the “any inducible AFL” groups, statistically significant differences between the groups were detected for OSA, P = 0.04, and persistent AF, P = 0.047. All other comparisons (gender, chronic obstructive pulmonary disease (COPD), asthma, hypertension, hyperlipidemia, diabetes, coronary artery disease and failure of at least one antiarrhythmic) yielded nonsignificant results. Similarly, for the comparison of “typical right AFL”, only OSA, P = 0.04, and persistent AF, P = 0.043, yielded statistically significant differences. Finally, for the comparison of “atypical AFL”, only female gender, P = 0.002, yielded a statistically significant difference. Table 4 shows all the descriptive statistics associated with the analyses on the categorical variables.
 Click to view | Table 4. Bivariate Analysis for Outcomes “Any, Typical, and Atypical Atrial Flutter” |
Due to the violation of normality for all continuous parameters of interest, non-parametric between-subjects statistical tests were performed on the current data. When comparing the “any inducible AFL” groups, statistically significant differences between the groups were detected for BMI, P = 0.015, and for left atrial size, P = 0.03. All other comparisons (age, EF, right atrial size, tricuspid regurgitation (TR) severity, and mitral regurgitation (MR) severity) yielded nonsignificant results. Similarly, for the comparison of “typical right AFL” groups, only BMI, P = 0.015, and left atrial size, P = 0.02, yielded statistically significant differences. Finally, for the comparison of “atypical AFL” groups, there were no significant differences detected. Table 5 shows all the descriptive statistics associated with the analyses on the continuous variables.
 Click to view | Table 5. Bivariate Analysis for Outcomes “Any, Typical, and Atypical Atrial Flutter” |
Multivariate analysis
The variables of gender, AF type, BMI, OSA, failure of at least one antiarrhythmic, left atrial size, right atrial size, TR severity, and MR severity were entered into a logistic regression model predicting for the binary outcome, “any inducible AFL.” Only OSA was significantly associated with the outcome, AOR = 1.92, 95% CI: 1.003 - 3.69, P = 0.049, when controlling for all the other variables. The findings for the other independent parameters are presented in Table 6.
 Click to view | Table 6. Multivariate Analysis for Outcome of “Any Inducible Atrial Flutter” |
Ablation of inducible AFL at the time of PVI
Out of the 100 patients that had inducible AFL at the time of PVI, 89 patients went on to have an additional ablation for AFL prior to completion of their procedure. Of the 11 patients that did not undergo an additional AFL ablation, nine had atypical AFL.
Recurrence of AF/AFL at 1 year
At 1 year, we observed the following rates of recurrence: 31% for AF, 10% for AFL, and 38% for either AF or AFL. Recurrence during the follow-up period was defined as either an ECG or outpatient monitor showing AF or AFL, or hospitalization for documented AF or AFL. There was no significant difference in the rates of recurrence of AF, AFL, or either AF/AFL at 1 year when accounting for the presence of inducible AFL, inducible AFL that was ablated and inducible AFL that was not ablated (Table 7).
 Click to view | Table 7. Recurrence of Atrial Fibrillation and Atrial Flutter at 1 Year |
| Discussion | ▴Top |
In our study the incidence of inducible AFL was 52%, with 82% of those patients experiencing typical inducible AFL and 18% experiencing atypical inducible AFL. These findings are consistent with those reported in the 2004 retrospective study that evaluated the clinical significance of inducible AFL during PVI, where the incidence of inducible AFL was approximately 65%, with 79% of those patients experiencing typical inducible AFL and 21% experiencing atypical inducible AFL [7]. There are a few potential reasons why the incidence of inducible AFL is high during PVI procedures. One possible explanation is that the electrical isolation of the pulmonary veins can create a pro-arrhythmic substrate that is conducive to the development of AFL. This may occur due to alterations in atrial conduction patterns, changes in refractory periods, or other factors that increase the likelihood of reentrant circuits forming in the atria.
Our study found that OSA, BMI, left atrial size, and persistent AF were significant risk factors for inducible AFL on bivariate analysis. However, on multivariate analysis, only OSA remained a significant risk factor. This finding is consistent with several studies that have reported an association between OSA and AFL. For instance, a study by Gupta et al in 2008 found that patients with OSA had a higher incidence of AFL compared to those without OSA (12.6% vs. 1.2%, P < 0.001). The authors suggested that OSA-induced changes in cardiac autonomic tone and atrial remodeling may contribute to the increased risk of AFL in OSA patients [8].
Similarly, Fein et al (2013) reported an increased incidence of AFL in OSA patients compared to those without OSA (23% vs. 12%, P = 0.03). The authors hypothesized that OSA-related hypoxemia and sympathetic activation may contribute to atrial remodeling and the development of AFL [9]. A more recent study by Lee et al (2019) investigated the association between OSA and AFL in patients who underwent catheter ablation for AF. The authors found that OSA was an independent predictor of AFL recurrence after ablation, with OSA patients having a higher risk of recurrence compared to those without OSA (hazard ratio: 2.02, 95% CI: 1.18 - 3.45, P = 0.01). The authors suggested that OSA-related hypoxemia and sympathetic activation may contribute to atrial remodeling and the development of AFL [10].
Additionally, we investigated the recurrence rates of AF and AFL at 1-year post-PVI and found that the overall rates of recurrence of AF, AFL, or a combination of both (AF/AFL) were 31%, 10%, and 38%, respectively. However, the presence of inducible AFL during PVI did not significantly affect the recurrence rates of AF, AFL, or AF/AFL at 1-year post-PVI. This differs from the study by Scharf et al [7], which showed that inducible AFL during PVI was predictive of symptomatic AFL during follow-up. However, our study excluded patients with a history of AFL, which likely explains this difference. Furthermore, there was no significant difference in the recurrence rates of AF or AFL when comparing patients who had inducible AFL that was successfully ablated during PVI to those who did not have inducible AFL.
Therefore, our study suggests that the presence of inducible AFL at the time of PVI may not significantly impact the recurrence rates of AF, AFL, or a combination of both at 1-year post-PVI. Additionally, our findings suggest that ablating inducible AFL during PVI may not provide a clinical benefit in terms of reducing the recurrence of AF or AFL during follow-up. However, it is important to note that our study was retrospective and had a 1-year follow-up period, which may limit the generalizability of our findings. Future prospective studies with longer follow-up periods and larger sample sizes are needed to confirm these findings and determine the clinical significance of inducible AFL during PVI in patients with different clinical characteristics.
Conclusions
In conclusion, the incidence of inducible AFL during PVI is high, with patients with OSA being at increased risk. However, the clinical significance of inducible AFL remains unclear, as our study found that it may not significantly affect the recurrence rates of AF or AFL at 1-year post-PVI. Furthermore, the successful ablation of inducible AFL during PVI may not provide clinical benefit in reducing the recurrence of AF or AFL. Therefore, more prospective studies with larger sample sizes and longer follow-up periods are needed to determine the clinical significance of inducible AFL during PVI in different patient populations.
Limitations
There are several limitations to our study that should be acknowledged. Firstly, our study design was retrospective, which may have introduced selection bias and limited the scope of our analysis. Secondly, although OSA was a central focus of our paper, we were not able to characterize OSA based on severity or treatment due to limited information in our dataset. This may have influenced our findings related to the association between OSA and AF/AFL. Lastly, we may have missed asymptomatic episodes of AF or AFL during follow-up, which could have affected our estimates of recurrence rates.
Acknowledgments
None to disclose.
Financial Disclosure
None to disclose.
Conflict of Interest
The authors report no financial relationships regarding the content herein.
Informed Consent
Study was exempt given the retrospective nature.
Author Contributions
Raj Baljepally: writing - review and editing, supervision. John Taylor: writing - original draft, methodology, conceptualization, formal analysis, writing - review and editing. Sohiub N. Assaf: writing - original draft, methodology, conceptualization, formal analysis, writing - review and editing. Abdallah N. Assaf: methodology, conception, and data acquisition. Eric Heidel: statistical analysis. William Mahlow: methodology, conception, supervision. All authors have participated in this work, reviewed it, and agreed with the content of the article.
Data Availability
The data supporting these findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Potpara TS, Polovina MM, Marinkovic JM, et al. Atrial fibrillation and flutter—different entities within the same disease? Data from the Euro Heart Survey. Herz. 2008;33(2):96-101.
doi - Boriani G, Biffi M, Diemberger I, Martignani C, Branzi A, Valzania C. Atrial fibrillation in patients with atrial flutter: Insights from a registry study of 407 consecutive patients. Journal of Cardiovascular Electrophysiology. 2010;21(11):1196-1202.
doi - Wang Y-C, Lee K-T, Hsueh S-K, et al. Coexistence of atrial fibrillation and atrial flutter and the risk of adverse outcomes: a nationwide cohort study. J Am Heart Assoc. 2019;8(8):e012139.
doi - Pontoppidan J, Nielsen JC, Poulsen SH, Jensen HK, Walfridsson H, Pedersen AK, Hansen PS. Prophylactic cavotricuspid isthmus block during atrial fibrillation ablation in patients without atrial flutter: a randomised controlled trial. Heart. 2009;95(12):994-999.
doi pubmed - Kim SH, Oh YS, Choi Y, Hwang Y, Kim JY, Kim TS, Kim JH, et al. Long-term efficacy of prophylactic cavotricuspid isthmus ablation during atrial fibrillation ablation in patients without typical atrial flutter: a prospective, multicentre, randomized trial. Korean Circ J. 2021;51(1):58-64.
doi pubmed pmc - Mesquita J, Ferreira AM, Cavaco D, Carmo P, Madeira M, Freitas P, Costa FM, et al. Impact of prophylactic cavotricuspid isthmus ablation in atrial fibrillation recurrence after a first pulmonary vein isolation procedure. Int J Cardiol. 2018;259:82-87.
doi pubmed - Scharf C, Veerareddy S, Ozaydin M, Chugh A, Hall B, Cheung P, Good E, et al. Clinical significance of inducible atrial flutter during pulmonary vein isolation in patients with atrial fibrillation. J Am Coll Cardiol. 2004;43(11):2057-2062.
doi pubmed - Gupta P, Patel P, Stradling JR, Shaw AM. Atrial fibrillation and sleep apnea: a common association of uncommon diseases. Journal of Clinical Sleep Medicine. 2008;4(6):583-586.
doi - Fein AS, Shvilkin A, Shah D, Haffajee CI, Das S, Kumar K, Kramer DB, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2013;62(4):300-305.
doi pubmed - Lee JH, Yoon YE, Park JK, Kim TH, Hwang C, Lee JH, Joung B. Impact of obstructive sleep apnea on the recurrence of atrial fibrillation after catheter ablation: a propensity score matching analysis. International Journal of Cardiology. 2019;278:277-283.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


