| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 14, Number 5, October 2023, pages 387-395
Association Between the Presence of Coronary Artery Disease or Peripheral Artery Disease and Left Ventricular Mass in Patients Who Have Undergone Coronary Computed Tomography Angiography
Tetsuro Tachibanaa, Yuhei Shigaa, Tetsuo Hirataa, Kohei Tashiroa, Sara Higashia, Yuto Kawahiraa, Yuta Katoa, Takashi Kuwanoa, Makoto Sugiharaa, Shin-ichiro Miuraa, b, c
aDepartment of Cardiology, Fukuoka University School of Medicine, Fukuoka, Japan
bDepartment of Cardiology, Fukuoka University Nishijin Hospital, Fukuoka, Japan
cCorresponding Author: Shin-ichiro Miura, Department of Cardiology, Fukuoka University School of Medicine, Jonan-ku, Fukuoka 814-0180, Japan
Manuscript submitted June 26, 2023, accepted August 9, 2023, published online October 21, 2023
Short title: Coronary or Peripheral Artery Disease and LVM
doi: https://doi.org/10.14740/cr1532
| Abstract | ▴Top |
Background: Left ventricular mass (LVM) is a critical marker of future cardiovascular risk. We determined the association between LVM measured by coronary computed tomography angiography (CCTA) and the presence of coronary artery disease (CAD) or peripheral artery disease (PAD) in patients who had undergone CCTA for screening of CAD.
Methods: We enrolled 1,307 consecutive patients (66 ± 12 years old, 49% males) who underwent CCTA for screening of CAD at the Fukuoka University Hospital (FU-CCTA registry), and either were clinically suspected of having CAD or had at least one cardiovascular risk factor. Patients with coronary stenosis of ≥ 50% by CCTA were diagnosed as CAD. Patients with an ankle brachial pressure index < 0.9 or who had already been diagnosed with PAD were considered to have PAD. Left ventricular mass index (LVMI), left ventricular ejection fraction (LVEF), end-diastolic volume (EDV) and end-systolic volume (ESV) were measured. The patients were divided into CAD (-) and CAD (+) or PAD (-) and PAD (+) groups.
Results: The prevalences of CAD and PAD in all patients were 50% and 4.8%, respectively. Age, %males, %hypertension (HTN), %dyslipidemia (DL), %diabetes mellitus (DM), %smoking and %chronic kidney disease in the CAD (+) group were significantly higher than those in the CAD (-) group. Age, %males, %HTN, %DM and %smoking in the PAD (+) group were significantly higher than those in the PAD (-) group. CAD was independently associated with LVMI (odds ratio (OR): 1.01, 95% confidence interval (CI): 1.01 - 1.02, P < 0.01) in addition to age, male, HTN, DL, DM, and smoking. PAD was also independently associated with LVMI (OR: 1.01, 95% CI: 1.0 - 1.02, P = 0.018) in addition to age, DM, and smoking.
Conclusions: LVMI determined by CCTA may be useful for predicting atherosclerotic cardiovascular diseases including both CAD and PAD, although there were considerable differences between %CAD and %PAD in all patients.
Keywords: Left ventricular hypertrophy; Computed tomography angiography; Coronary artery disease; Peripheral artery disease
| Introduction | ▴Top |
Coronary computed tomography angiography (CCTA) is widely available in hospitals in Japan, allowing the noninvasive assessment of coronary artery stenosis, calcification, and plaque [1, 2]. CCTA can also be used to measure left ventricular ejection fraction (LVEF) and left ventricular mass (LVM) with the use of software [3, 4].
Left ventricular hypertrophy (LVH) is a predictor of cardiovascular morbidity and mortality [5-12], and the improvement of LVH reduces the subsequent cardiovascular risk [10, 11]. The Framingham study showed that an increase in LVM of 50 g per height, as measured by echocardiography, was associated with an increased risk of coronary artery disease (CAD) [9].
The ankle brachial index (ABI) is an indicator of generalized vascular atherosclerosis because lower levels have been associated with higher rates of concomitant coronary and cerebrovascular diseases, and with the presence of cardiovascular risk factors [13-18]. A low ABI has been related to an increased incidence of mortality (total and cardiovascular), myocardial infarction and stroke. These increased relative risks have been shown to be independent of baseline cardiovascular disease and risk factors, suggesting that ABI might play an independent role in predicting cardiovascular events. Some studies have shown a high prevalence of LVH in patients at their first diagnosis of peripheral artery disease (PAD) [19]. The prevalence of echo LVH when LVM was indexed to body surface area (BSA) was 50%. There is already a high prevalence of LVH in patients at their first diagnosis of PAD [20]. Therefore, LVH is common enough in PAD patients to potentially make a major contribution to cardiac death.
We have been studying the risk factors for CAD at the Fukuoka University Hospital for the primary prevention of CAD using the Fukuoka University CCTA registry (FU-CCTA registry) [21-24]. Therefore, we determined the association between LVM measured by CCTA and the presence of CAD or PAD in patients who have undergone CCTA for screening of CAD.
| Materials and Methods | ▴Top |
Study subjects
We performed a cross-sectional study and enrolled 1,307 consecutive patients who underwent CCTA for screening of CAD at the Fukuoka University Hospital (FU-CCTA registry) and either were clinically suspected of having CAD or had at least one cardiovascular risk factor. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. The study was approved by the Fukuoka University Ethics Committee and was conducted after written consent was obtained from the subjects (#09-10-02).
Evaluation of coronary artery stenosis and PAD
Coronary artery stenosis was evaluated by constructing a volume-rendered image (Aquilion ONE, Canon Medical Systems USA, Inc., Tustin, CA) and evaluating the degree of lumen stenosis with multiplanar images. Coronary stenosis of ≥ 50% by CCTA was considered to be CAD. The severity of coronary artery stenosis was determined by the number of branches with significant coronary stenosis, the coronary artery calcification (CAC) score, and the Gensini score. An analysis of cardiac function using the Aquilion ONE was used to automatically trace both diastolic and systolic cardiac function, and LVM, LVEF, left ventricular end-diastolic volume (LVEDV), and left ventricular end-systolic volume (LVESV) were measured. The left ventricular mass index (LVMI) was calculated by dividing LVM by the BSA in each patient. Patients with ABI < 0.9 or who had already been diagnosed with PAD were considered to have PAD.
Assessment of cardiovascular risks
All patients were evaluated with regard to body mass index (BMI), BSA, family history of CAD, smoking history, blood pressure (BP), hypertension (HTN), diabetes mellitus (DM), hemoglobin A1c (HbA1c), and fasting blood glucose (FBG). In addition, the presence of dyslipidemia (DL), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), non-HDL-C, chronic kidney disease (CKD), and estimated glomerular filtration rate (eGFR) were assessed.
Medications
Patients were being treated with an angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker (ACEI/ARB), β-blocker, calcium channel blocker (CCB), and/or diuretic (DU) for HTN. They were taking statins or eicosapentaenoic acid (EPA) for DL, and an α-glucosidase inhibitor (α-GI), biguanide, dipeptidyl peptidase-4 inhibitor (DPP4I), insulin, sulfonylurea (SU) or thiazolizine for DM.
Statistical analysis
Statistical analyses were performed using Excel (SSRI, Tokyo, Japan) at Fukuoka University (Fukuoka, Japan), and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). Continuous variables are shown as the mean ± standard deviation (SD). Categorical and continuous variables were compared between groups using a Chi-squared analysis and t-tests, respectively. Univariable and multivariable analyses of predictors for the presence of CAD or PAD were used to identify independent variables. We used a logistic regression model to examine multiple independent variables with the presence of CAD or PAD as the dependent variable. For the selection of independent variables, we selected from items that are believed to have a clinical impact in the presence of CAD or PAD (risk factors for atherosclerotic cardiovascular diseases) and added LVMI and some medications (ACEI/ARB and β-blocker) that affect LVMI.
| Results | ▴Top |
Patient characteristics in the non-CAD and CAD groups
Patient characteristics in the non-CAD and CAD groups are shown in Table 1. A total of 1,308 patients were enrolled: 658 in the non-CAD group and 650 in the CAD group. Age was 62 ± 13 years in the non-CAD group and 69 ± 10 years in the CAD group, and this difference was significant. The gender difference was also significant; 41% of the non-CAD group were male, compared to 57.2% of the CAD group. Similarly, there were significant differences in the prevalences of smoking (29.6% vs. 38.8%, P < 0.05), HTN (55% vs. 75.7%, P < 0.05), DM (18.5% vs. 32.2%, P < 0.05), DL (62.2% vs. 73.3%, P < 0.05) and CKD (22.9% vs. 32%, P < 0.05). In the evaluation of coronary artery, significant differences were found in the number of diseased branches, the CAC score, and the Gensini score. In terms of treatment, there were significant differences in the use of ACEI/ARB, β-blocker, CCB, statin, SU, α-GI, biguanide, and DPP4I.
 Click to view | Table 1. Patient Characteristics in the Non-CAD and CAD Groups |
Patient characteristics in the non-PAD and PAD groups
Patient characteristics in the non-PAD and PAD groups are shown in Table 2. Age was 65 ± 12 years in the non-PAD group and 70 ± 10 years in the PAD group, and this difference was significant. The gender difference was also significant: 61.9% of the PAD group were male, compared to 48.6% of the non-PAD group. Similarly, there were significant differences in the prevalences of smoking (33.5% vs. 52.4%, P < 0.05), HTN (64.5% vs. 79.4%, P < 0.05) and DM (24.4% vs. 46%, P < 0.05). In the evaluation of coronary arteries, significant differences were observed in the number of diseased vessels, the CAC score, and the Gensini score. Significant differences were also observed in treatment with SU, DPP4I, and insulin.
 Click to view | Table 2. Patient Characteristics in the Non-PAD and PAD Groups |
LV profiles in the non-CAD and CAD groups
LV function on CT was examined in the non-CAD and CAD groups (Fig. 1). Although LVEF and LVESV were not significantly different between the two groups, LVMI in the CAD group was significantly increased (71.6 ± 22.6 g/m2 vs. 65.0 ± 18.2 g/m2, P < 0.05). In addition, LVEDV in the non-CAD group was significantly lower than that in the CAD group (111.7 ± 34.0 mL vs. 116.7 ± 31.3 mL, P < 0.05).
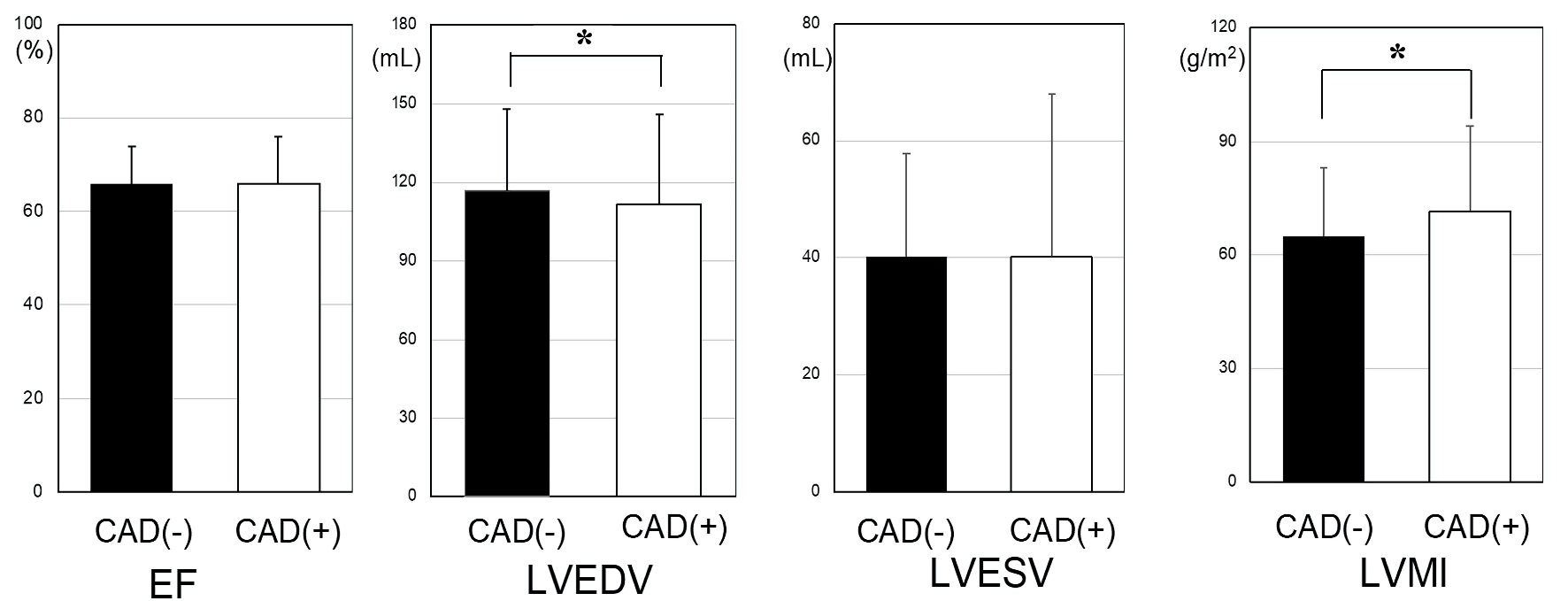 Click for large image | Figure 1. LV profiles in the non-CAD and CAD groups. *P < 0.05. CAD: coronary artery disease; LV: left ventricular; LVMI: left ventricular mass index; LVEF: left ventricular ejection fraction; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume. |
LV profiles in the non-PAD and PAD groups
LV function was examined by CT in the non-PAD and PAD groups (Fig. 2). There were no significant differences between the two groups in LV profiles, although LVMI in the PAD (+) group tended to be higher than that in the PAD (-) group (75.9 ± 35.1 g/m2 vs. 67.8 ± 19.6 g/m2, P = 0.074).
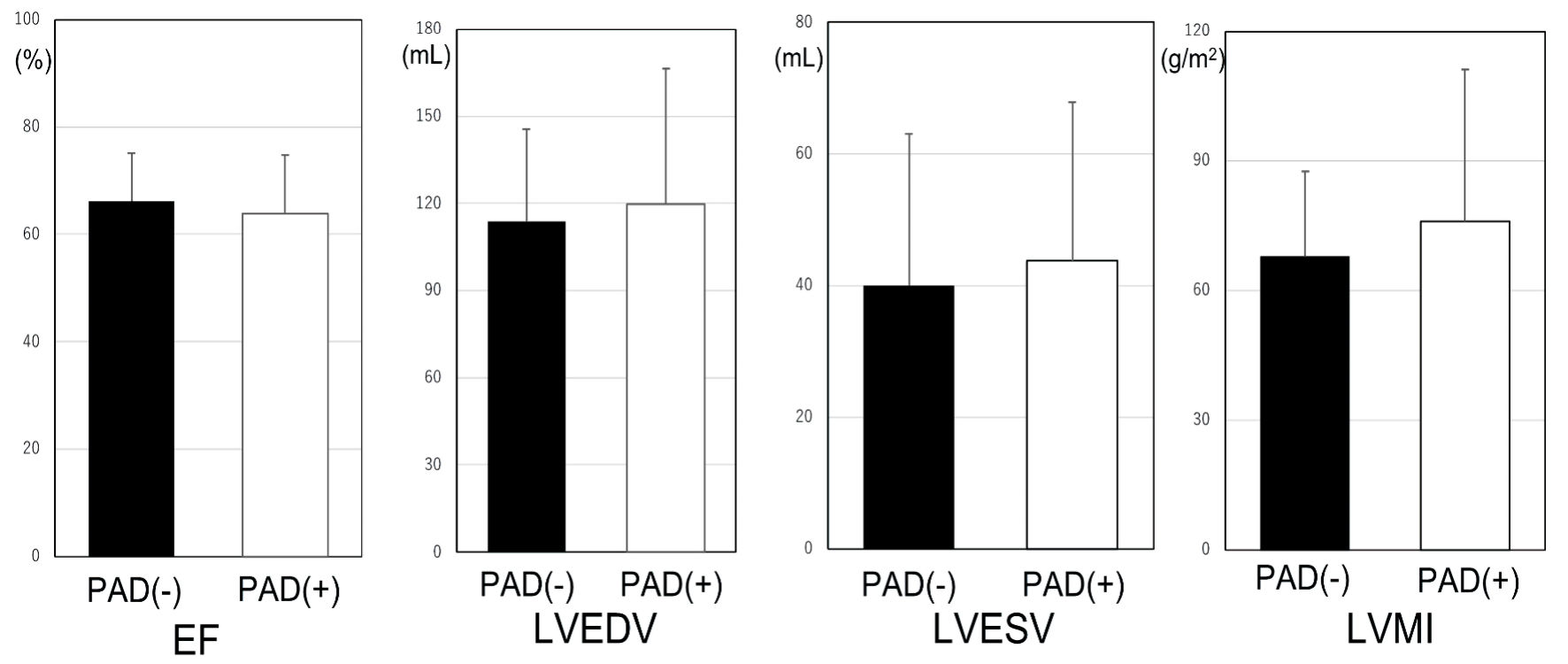 Click for large image | Figure 2. LV profiles in the non-PAD and PAD groups. PAD: peripheral artery disease; LV: left ventricular; LVMI: left ventricular mass index; LVEF: left ventricular ejection fraction; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume. |
LVMI in the non-CAD/non-PAD, CAD/non-PAD, non-CAD/PAD and CAD/ PAD groups
LVMI in the CAD/non-PAD and CAD/PAD groups were significantly higher than that in the non-CAD/non-PAD group (Table 3).
 Click to view | Table 3. LVMI in the Non-CAD/Non-PAD, CAD/Non-PAD, Non-CAD/PAD and CAD/PAD Groups |
Univariable and multivariable analyses of predictors for the presence of CAD
A univariable analysis was performed on predictors for the presence of CAD (Table 4). Age, male, HTN, ACEI/ARB, β-blocker, DL, DM, smoking and CKD in addition to LVMI were significantly associated with the presence of CAD. A multivariable analysis of predictors for CAD is also shown in Table 4. The presence of CAD was independently associated with LVMI (odds ratio (OR): 1.01, 95% confidence interval (CI): 1.01 - 1.02, P < 0.01) in addition to age, male, HTN, DL, DM and smoking.
 Click to view | Table 4. Univariable and Multivariable Analyses of Predictors for the Presence of CAD |
Univariable and multivariable analyses of predictors for the presence of PAD
A univariable analysis was performed with predictors for PAD (Table 5). The results showed associations between the presence of PAD and age, male, HTN, DM, smoking or LVMI. The presence of PAD was independently associated with LVMI (OR: 1.01, 95% CI: 1.0 - 1.02, P = 0.018) in addition to age, DM and smoking by a multivariable analysis (Table 5).
 Click to view | Table 5. Univariable and Multivariable Analyses of Predictors for the Presence of PAD |
| Discussion | ▴Top |
In this study, we analyzed the association between LVMI and the presence of CAD or PAD. An increased LVMI is associated with all-cause mortality, arrhythmic death, and sudden death in patients with CAD [25]. In addition, the Framingham study showed that an increase in LVM over time by echocardiography in a healthy population is a risk factor for major adverse cardiovascular events (MACEs) [7, 9]. These previous studies suggest that LVH may be useful for predicting subsequent MACEs and all-cause mortality. Thus, we hypothesized that LVMI obtained at the time of CCTA was associated with CAD or PAD because atherosclerotic cardiovascular diseases such as CAD and PAD are MACEs. Finally, we found that the presence of CAD or PAD was associated with LVMI in patients with suspected CAD or at least one cardiovascular risk at the time of CCTA for screening of CAD.
Another important issue in this study was the association between PAD and LVMI. PAD was also independently associated with LVMI by a multivariable analysis. It has been shown that, among patients with CKD, those with higher BMI and LVMI tend to have an abnormal ABI [26]. It has also been suggested that adding ABI to the Framingham risk score may provide a more accurate assessment of all-cause mortality, including cardiovascular death [27]. Several studies have shown that LVH is strongly associated with cardiovascular risk and mortality [9, 28]. Furthermore, it has been suggested that the presence of LVH is associated with the risk of cardiovascular death in patients with PAD, and that the prevalence of LVH is increased by 29% when patients have PAD in addition to HTN and angina pectoris [29]. A previous study showed that 50% of patients with PAD had LVH, and that LVH is common in patients with PAD [24]. Our study in patients who underwent CCTA for screening of CAD is also consistent with the results reported previously.
Since DM, HTN, and DL are already known risk factors for CAD, we were also able to confirm that DM, HTN, and DL were associated with CAD. Therefore, it is assumed that the subjects of this study are not a special population, and we believe that this is worth analyzing. HTN, DL, smoking, and DM are also well-known risk factors for PAD [30]. However, in this study, while smoking and DM were associated with PAD, HTN and DL were not. In a previous study [30], the study population consisted of subjects from the healthy general population. In contrast, in this study, the population consisted of patients with cardiovascular risk or suspected CAD. The proportion of patients with cardiovascular risks in their background in the present study was relatively high. Therefore, it is possible that some risk factors were not associated with the presence of PAD. On the other hand, older age, DM, and smoking might be more strongly associated with PAD than other risk factors. The 2007 TASC II consensus document lists the OR for various risk factors of symptomatic PAD [31]. The OR of DM and smoking are roughly between 3 and 4, whereas those for HTN and DL are between approximately 1.5 and 2. There have been no reports of OR for risk factors related to asymptomatic PAD. While our study includes patients with asymptomatic conditions and the patient backgrounds differ, which makes direct comparisons challenging; HTN and DL can be considered to have a lower attributable risk for PAD compared to DM and smoking. In addition, the PAD group consisted of only 63 individuals, suggesting low statistical power.
There was a gender difference in the association between CAD and LVMI. It has been shown that males are at higher risk for CAD compared to females [32] even after adjustment for cardiovascular risk factors. Although females are more likely to develop PAD than males in all age groups [33], the present study found no gender association by a multiple logistic analysis in patients with PAD. Additional studies will be needed to resolve this issue.
Study limitations
First, patients who had at least one cardiovascular risk factor or who were clinically suspected of having CAD were enrolled in this study. Although the number of cases of CAD was sufficient, the number of cases of PAD was small. This may have contributed to the difficulty of a statistical comparison. Second, it was not possible to evaluate patients over time, and the influence of changes in LVH could not be examined as that in a prospective study, because CAD and PAD were evaluated at the time of CCTA. Third, PAD was evaluated by ABI and not by angiography or contrast CT. Therefore, the lesion site could not be evaluated.
Conclusions
LVMI, as measured by CCTA, was associated with atherosclerotic cardiovascular diseases, including CAD and PAD, and may be a useful imaging biomarker.
Acknowledgments
The authors thank the members of the Department of Cardiology, Fukuoka University, for their valuable suggestions and discussions.
Financial Disclosure
The authors have no financial disclosure or funding.
Conflict of Interest
The authors have no conflict of interest.
Informed Consent
The study was approved by the Fukuoka University Ethics Committee and was conducted after written informed consent was obtained from the subjects.
Author Contributions
Conceptualization: TT and YS. Methodology: YS and KT. Data collection: TT, YS, KT, SH and Y. Kawahira. Software: YS and KT. Formal analysis: TT and YS. Investigation: TH, TT and Y. Kawahira. Writing - original draft preparation: TT. Writing - review and editing: TH, Y. Kato, and TK. Supervision: MS and SM. All authors have read and agreed to the published version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Rumberger JA, Sheedy PF, 3rd, Breen JF, Schwartz RS. Coronary calcium, as determined by electron beam computed tomography, and coronary disease on arteriogram. Effect of patient's sex on diagnosis. Circulation. 1995;91(5):1363-1367.
doi pubmed - Achenbach S. Detection of coronary stenoses by multidetector computed tomography: it's all about resolution. J Am Coll Cardiol. 2004;43(5):840-841.
doi pubmed - Abbara S, Chow BJ, Pena AJ, Cury RC, Hoffmann U, Nieman K, Brady TJ. Assessment of left ventricular function with 16- and 64-slice multi-detector computed tomography. Eur J Radiol. 2008;67(3):481-486.
doi pubmed - Muhlenbruch G, Das M, Hohl C, Wildberger JE, Rinck D, Flohr TG, Koos R, et al. Global left ventricular function in cardiac CT. Evaluation of an automated 3D region-growing segmentation algorithm. Eur Radiol. 2006;16(5):1117-1123.
doi pubmed - Takagi Y, Ehara S, Okuyama T, Shirai N, Yamashita H, Sugioka K, Kitamura H, et al. Comparison of determinations of left atrial volume by the biplane area-length and Simpson's methods using 64-slice computed tomography. J Cardiol. 2009;53(2):257-264.
doi pubmed - Aronow WS, Ahn C, Kronzon I, Koenigsberg M. Congestive heart failure, coronary events and atherothrombotic brain infarction in elderly blacks and whites with systemic hypertension and with and without echocardiographic and electrocardiographic evidence of left ventricular hypertrophy. Am J Cardiol. 1991;67(4):295-299.
doi pubmed - Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort. The Framingham Heart Study. Ann Intern Med. 1989;110(2):101-107.
doi pubmed - Aronow WS, Koenigsberg M, Schwartz KS. Usefulness of echocardiographic left ventricular hypertrophy in predicting new coronary events and atherothrombotic brain infarction in patients over 62 years of age. Am J Cardiol. 1988;61(13):1130-1132.
doi pubmed - Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561-1566.
doi pubmed - Verdecchia P, Angeli F, Borgioni C, Gattobigio R, de Simone G, Devereux RB, Porcellati C. Changes in cardiovascular risk by reduction of left ventricular mass in hypertension: a meta-analysis. Am J Hypertens. 2003;16(11 Pt 1):895-899.
doi pubmed - Dzau VJ. Tissue renin-angiotensin system in myocardial hypertrophy and failure. Arch Intern Med. 1993;153(8):937-942.
pubmed - Kishi S, Magalhaes TA, George RT, Dewey M, Laham RJ, Niinuma H, Friedman LA, et al. Relationship of left ventricular mass to coronary atherosclerosis and myocardial ischaemia: the CORE320 multicenter study. Eur Heart J Cardiovasc Imaging. 2015;16(2):166-176.
doi pubmed pmc - Weatherley BD, Nelson JJ, Heiss G, Chambless LE, Sharrett AR, Nieto FJ, Folsom AR, et al. The association of the ankle-brachial index with incident coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study, 1987-2001. BMC Cardiovasc Disord. 2007;7:3.
doi pubmed pmc - Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelmark M, Polak JF, Powe NR, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol. 1999;19(3):538-545.
doi pubmed - Leng GC, Fowkes FG, Lee AJ, Dunbar J, Housley E, Ruckley CV. Use of ankle brachial pressure index to predict cardiovascular events and death: a cohort study. BMJ. 1996;313(7070):1440-1444.
doi pubmed pmc - Hooi JD, Kester AD, Stoffers HE, Rinkens PE, Knottnerus JA, van Ree JW. Asymptomatic peripheral arterial occlusive disease predicted cardiovascular morbidity and mortality in a 7-year follow-up study. J Clin Epidemiol. 2004;57(3):294-300.
doi pubmed - Ogren M, Hedblad B, Isacsson SO, Janzon L, Jungquist G, Lindell SE. Non-invasively detected carotid stenosis and ischaemic heart disease in men with leg arteriosclerosis. Lancet. 1993;342(8880):1138-1141.
doi pubmed - van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DA, Witteman JC. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation. 2004;109(9):1089-1094.
doi pubmed - Belch JJ, Topol EJ, Agnelli G, Bertrand M, Califf RM, Clement DL, Creager MA, et al. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med. 2003;163(8):884-892.
doi pubmed - Wright GA, Ang DS, Stonebridge PA, Belch JJ, Struthers AD. Left ventricular hypertrophy is present in one-half of newly-diagnosed peripheral arterial disease patients. J Hypertens. 2007;25(2):463-469.
doi pubmed - Nakamura A, Miura S, Shiga Y, Norimatsu K, Miyase Y, Suematsu Y, Mitsutake R, et al. Is pentraxin 3 a biomarker, a player, or both in the context of coronary atherosclerosis and metabolic factors? Heart Vessels. 2015;30(6):752-761.
doi pubmed - Norimatsu K, Miura S, Suematsu Y, Shiga Y, Miyase Y, Nakamura A, Yamada M, et al. Associations between glycated albumin or hemoglobin A1c and the presence of coronary artery disease. J Cardiol. 2015;65(6):487-493.
doi pubmed - Yano M, Miura S, Shiga Y, Miyase Y, Suematsu Y, Norimatsu K, Nakamura A, et al. Association between smoking habits and severity of coronary stenosis as assessed by coronary computed tomography angiography. Heart Vessels. 2016;31(7):1061-1068.
doi pubmed - Norimatsu K, Kuwano T, Miura SI, Shimizu T, Shiga Y, Suematsu Y, Miyase Y, et al. Significance of the percentage of cholesterol efflux capacity and total cholesterol efflux capacity in patients with or without coronary artery disease. Heart Vessels. 2017;32(1):30-38.
doi pubmed - Turakhia MP, Schiller NB, Whooley MA. Prognostic significance of increased left ventricular mass index to mortality and sudden death in patients with stable coronary heart disease (from the Heart and Soul Study). Am J Cardiol. 2008;102(9):1131-1135.
doi pubmed pmc - Chen SC, Lee WH, Hsu PC, Huang JC, Lee CS, Lin TH, Voon WC, et al. Association of body mass index and left ventricular mass index with abnormally low and high ankle-brachial indices in chronic kidney disease. Hypertens Res. 2016;39(3):166-170.
doi pubmed - Ankle Brachial Index Collaboration, Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300(2):197-208.
doi pubmed pmc - Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham Heart Study. Ann Intern Med. 1988;108(1):7-13.
doi pubmed - Sukhija R, Aronow WS, Kakar P, Levy JA, Lehrman SG, Babu S. Prevalence of echocardiographic left ventricular hypertrophy in persons with systemic hypertension, coronary artery disease, and peripheral arterial disease and in persons with systemic hypertension, coronary artery disease, and no peripheral arterial disease. Am J Cardiol. 2005;96(6):825-826.
doi pubmed - Joosten MM, Pai JK, Bertoia ML, Rimm EB, Spiegelman D, Mittleman MA, Mukamal KJ. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308(16):1660-1667.
doi pubmed pmc - Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5-S67.
doi pubmed - Sharma SP, Manintveld OC, Budde RPJ, Hirsch A, Lenzen MJ, Galema TW. Gender differences in patients with stable chest pain. Am J Cardiol. 2022;171:84-90.
doi pubmed - Pabon M, Cheng S, Altin SE, Sethi SS, Nelson MD, Moreau KL, Hamburg N, et al. Sex differences in peripheral artery disease. Circ Res. 2022;130(4):496-511.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


