| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 14, Number 6, December 2023, pages 429-436
The Association Between Non-Clinically Apparent Liver Fibrosis and Pulmonary Arterial Hypertension in Hispanic Patients
M. Ammar Kalasa, Yacoub Khatabb, Gian Galuraa, Haider Alkhateebc, Debabrata Mukherjeec , Hernando Garciad, Marc Zuckermana, Nils Patrick Nickele, f
aDivision of Gastroenterology, Texas Tech University Health Sciences Center El Paso, El Paso, TX 79905, USA
bPaul L. Foster School of Medicine, Texas Tech University Health Sciences Center El Paso, El Paso, TX 79905, USA
cDivision of Cardiology, Texas Tech University Health Sciences Center El Paso, El Paso, TX 79905, USA
dDivision of Pulmonary and Critical Care Medicine, Mount Sinai Medical Center, Miami Beach, FL 33140, USA
eDivision of Pulmonary and Critical Care Medicine, Texas Tech University Health Sciences Center El Paso, El Paso, TX 79905, USA
fCorresponding Author: Nils Nickel, Division of Pulmonary and Critical Care Medicine, Texas Tech University Health Sciences Center El Paso, El Paso, TX 79905, USA
Manuscript submitted August 30, 2023, accepted October 3, 2023, published online December 9, 2023
Short title: Association Between Liver Fibrosis and PAH
doi: https://doi.org/10.14740/cr1565
| Abstract | ▴Top |
Background: Pulmonary arterial hypertension (PAH) is a deadly cardiopulmonary disease with multi-organ involvement including impaired liver function. Liver dysfunction in PAH is poorly understood but significantly associated with morbidity and mortality. Hispanics have a significantly higher prevalence of non-alcoholic fatty liver disease (NAFLD) and evidence of more advanced disease in comparison to other ethnic groups. The clinical impact of NAFLD in Hispanic PAH patients is unknown. We aimed to investigate the impact of a validated scoring system, non-alcoholic fatty liver disease fibrosis score (NFS), to predict the degree of liver fibrosis in a Hispanic PAH population and its relationship to hemodynamics, functional class, and outcomes.
Methods: A retrospective review of all treatment-naive Hispanic patients with group I World Health Organization (WHO) pulmonary hypertension (PH) at a single academic center between February 2016 and March 2021 was performed. Patients with history of substance or alcohol abuse, non-group I WHO PH, pre-existent liver disease, chronic kidney disease, atrial fibrillation, thyroid disease, and warfarin use were excluded from the study. The diagnosis of group I WHO PH was determined by cardiac catheterization after the exclusion of other etiologies. NFS was calculated for each patient and correlated with functional capacity, hemodynamics, N-terminal-pro-B-type natriuretic peptide (NT-proBNP), and survival.
Results: A total of 96 Hispanic patients were included in our study. The median age of patients in our cohort was 49 years (interquartile range: 15) and 69% of our cohort were females. Higher NFSs indicating advanced hepatic fibrosis (F3-F4) were found to correlate with elevated right-sided cardiac filling pressures (r = 0.27, P = 0.03), elevated levels of NT-proBNP (r = 0.32, P = 0.01), lower 6-minute walk distance (6MWD) (r = -0.49, P = 0.001), lower functional capacity (World Health Organization functional class, WHO-FC, r = -0.35, P = 0.051), a higher prevalence of diabetes (21.1% versus 51.9%, P = 0.001), a higher prevalence of risk factors for metabolic syndrome (81.5% versus 65.0%, P = 0.035), and worse 5-year survival rates.
Conclusion: In Hispanic patients with PAH, NFSs correlate with the degree of right-sided pressure overload. In addition, advanced NFSs were independently associated with lower 5-year survival rates and added prognostic information to other established risk parameters in PAH. This study suggests that screening for liver disease in this vulnerable patient population can aid in earlier detection leading to discussion of lifestyle modifications and possible escalation of PAH-targeted therapies, thus leading to potential improvement in survival rates.
Keywords: Pulmonary arterial hypertension; Liver fibrosis; Non-alcoholic fatty liver disease fibrosis score; Non-invasive assessment
| Introduction | ▴Top |
Pulmonary hypertension (PH) is defined as a mean pulmonary arterial pressure (mPAP) greater than 20 mm Hg without exertion. This is confirmed by right heart catheterization [1]. Hemodynamically, PH can be broadly classified into three groups: pre-capillary (pulmonary arterial hypertension (PAH)), post-capillary, or mixed. Pre-capillary and post-capillary PH can be differentiated based on the pulmonary vascular resistance (≥ 3 Woods unit in pre-capillary PH) and pulmonary capillary wedge pressure (PCWP) (less than 15 mm Hg for pre-capillary). Clinically, PH can be classified into five subgroups: group 1 is PAH, group 2 is PH due to left heart disease, group 3 is PH due to pulmonary disease, group 4 is PH due to chronic thromboembolic disease, and group 5 is miscellaneous causes such as complex congenital heart disease [2]. PAH is a pre-capillary form of PH which has recently been regarded as a multisystem disease rather than a purely pulmonary entity [3].
Due to the elevations in right ventricular (RV) pressures in PH, upstream clinical and biochemical effects are expected. The liver is one of the first organs affected by elevated RV pressures resulting in abnormalities in liver biochemical tests and ascites [4]. The lung-liver relationship is reciprocal: elevated right-sided filling pressures from PH are associated with congestive hepatopathy. Similarly, liver disease clearly has implications on the anatomy and function of the pulmonary circulation, as seen in porto-pulmonary hypertension, hepato-pulmonary syndrome [5], and Fontan physiology causing pulmonary arteriovenous malformations [6].
The relation between PH and non-clinically apparent liver fibrosis has not been evaluated directly in the past. In this study, we retrospectively reviewed a cohort of Hispanic PAH patients in a single academic center and correlate hemodynamic parameters, functional capacity, biochemical variables, and outcomes with the non-alcoholic fatty liver disease (NAFLD) fibrosis scores (NFSs).
This study aimed to assess the prevalence of liver fibrosis based on non-invasive NFSs in Hispanic patients with PAH and the relationship of fibrosis with hemodynamic, functional, and biochemical variables of disease severity as well as 5-year survival.
| Materials and Methods | ▴Top |
A single-center retrospective cohort study consisting of 96 treatment-naive Hispanic patients with World Health Organization (WHO) group 1 PH (PAH) from the PAH registry at Texas Tech University Health Sciences Center El Paso (TTUHSCEP) pulmonary hypertension clinic and University Medical Center (UMC) of El Paso between February 2016 and March 2021 was performed. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee (IRB # E21164). Diagnosis of PAH was made by right heart catheterization based on most up to date clinical guidelines at the time of diagnosis. Exclusion criteria were: non-WHO group 1 PH, non-Hispanics, documented pre-existing liver disease (based on ICD-10 codes and physician documentation) (Supplementary Material 1, www.cardiologyres.org), patients with viral hepatitis, porto-pulmonary hypertension, a history of substance or alcohol abuse, chronic kidney disease, atrial fibrillation, thyroid disease, and warfarin use. Each chart was independently reviewed by two investigators and was validated by the treating PH physician. In all patients, the diagnosis of PAH was made by right heart catheterization and exclusion of other forms of PH by laboratory studies, echocardiography, pulmonary function testing, computed tomography angiography, and ventilation-perfusion scanning. Ethnicity was determined by self-reporting during clinic encounters. All patients were seen and treated at the UMC El Paso and TTUHSCEP pulmonary hypertension clinic. Patients were monitored by regular outpatient visits for a median of 3.6 years (range 3 - 60 months). Survival status was censored on May 31, 2022. The primary end-point of this study was 5-year survival after the diagnosis of PAH.
NFSs were calculated using the following formula: 1.675 + (0.037 × age (years)) + (0.094 × body mass index (kg/m2)) + (1.13 × diabetes (yes = 1, no = 0)) + (0.99 × AST/ALT ratio) - (0.013 × platelet (× 109/L)) - (0.66 × albumin (g/dL)). NFSs below -1.455 correlate with histological fibrosis stage F0-F2 with a negative predictive value of 88-93% for advanced fibrosis. NFSs between -1.455 and 0.675 are considered indeterminate, and NFS > 0.675 correlates with F3-F4 histological fibrosis with a positive predictive value of 82-90% for advanced fibrosis. The staging of the fibrosis was based on the histological Brunt criteria. The criteria include five separate stages: stage 0 = absent fibrosis, stage 1 = portal or perisinusoidal fibrosis, stage 2 = portal/periportal and perisinusoidal fibrosis, stage 3 = bridging or septal fibrosis, and stage 4 = cirrhosis [7]. Patients in this cohort were divided based on NFSs. Group 1 was considered negative for advanced fibrosis based on an NFS < -1.455. Group 2 was considered indeterminate if the NFS was between -1.455 and 0.675, based on ambiguity of the clinical relevance of these scores. Group 3 was considered high risk for fibrosis if the NFS was > 0.675. NFSs (one score calculated per patient) were calculated within 3 months of the initial right cardiac catheterization, functional class assessment, and 6-minute walk distance.
Patients’ records were reviewed for any abdominal imaging obtained (ultrasonography, computed tomography, or liver transient elastography). However, the majority of patients did not have imaging studies and hence this variable was not included in the analysis.
All data are reported as absolute numbers, percentages, mean (standard deviation (SD)), or median (interquartile range (IQR)), as indicated. The relationship between NFSs and baseline variables was assessed using Mann-Whitney U testing for continuous variables and Chi-square testing for nominal or categorical variables. Correlations between continuous variables were assessed using the Pearson correlation coefficient. Spearman rank correlation was used to assess the correlation for nominal and categorical variables. Kaplan-Meier survival analysis was used to estimate overall survival in patients with low and high NFSs. Log-rank testing was used to assess the statistical significance between both groups. Cox regression analysis was used to identify non-invasive predictors of death during follow-up. All variables were tested for normality of distribution using Kolmogorov-Smirnov testing. Variables without normal distribution were transformed into their natural logarithm before Cox analysis. A P-value of less than 0.05 was considered statistically significant. All calculations and graphics were done using SPSS 27 (IBM), Excel (Microsoft), and Graph pad (8.0.0 GraphPad software, San Diego, CA).
| Results | ▴Top |
Out of a database with 236 patients with PAH, we identified 96 treatment-naive Hispanic patients (69% females) with PAH who met inclusion criteria (Fig. 1). Median age was 49 years (IQR: 15). Estimated median survival of the entire cohort was 3.9 years with survival percentages of 89.5% at 1 year, 77.4% at 3 years, and 68.7% at 5 years, respectively. The clinical characteristics and NFSs are listed in Table 1. The majority of patients in our cohort (37.5%) had F0-F2 stage of fibrosis according to the NFS. Twenty-eight percent of patients had an NFS (F3-F4) suggestive of advanced liver fibrosis. The remainder of the patients (34.4%) had an indeterminate NFS. Patients with advanced fibrosis score were older (48 versus 59 years, P = 0.05), predominantly male (45% versus 14% in the mild fibrosis group, P < 0.05), had a significantly worse WHO functional class (WHO-FC) (2.4 versus 2.9, P < 0.05), 6MWD (288 versus 399 m, P < 0.05), higher N-terminal-pro-B-type natriuretic peptide (NT-proBNP) levels (1,050 versus 2,323, P < 0.05), elevated right-sided filling pressures (mean right atrial pressure (mRAP) 11.5 versus 8 mm Hg, P < 0.05), a higher prevalence of diabetes (21.1% versus 51.9%, P = 0.001), and a higher prevalence of risk factors for metabolic syndrome (81.5% versus 65.0%, P = 0.035). The correlation between the NFS and patient variables is depicted in Table 2.
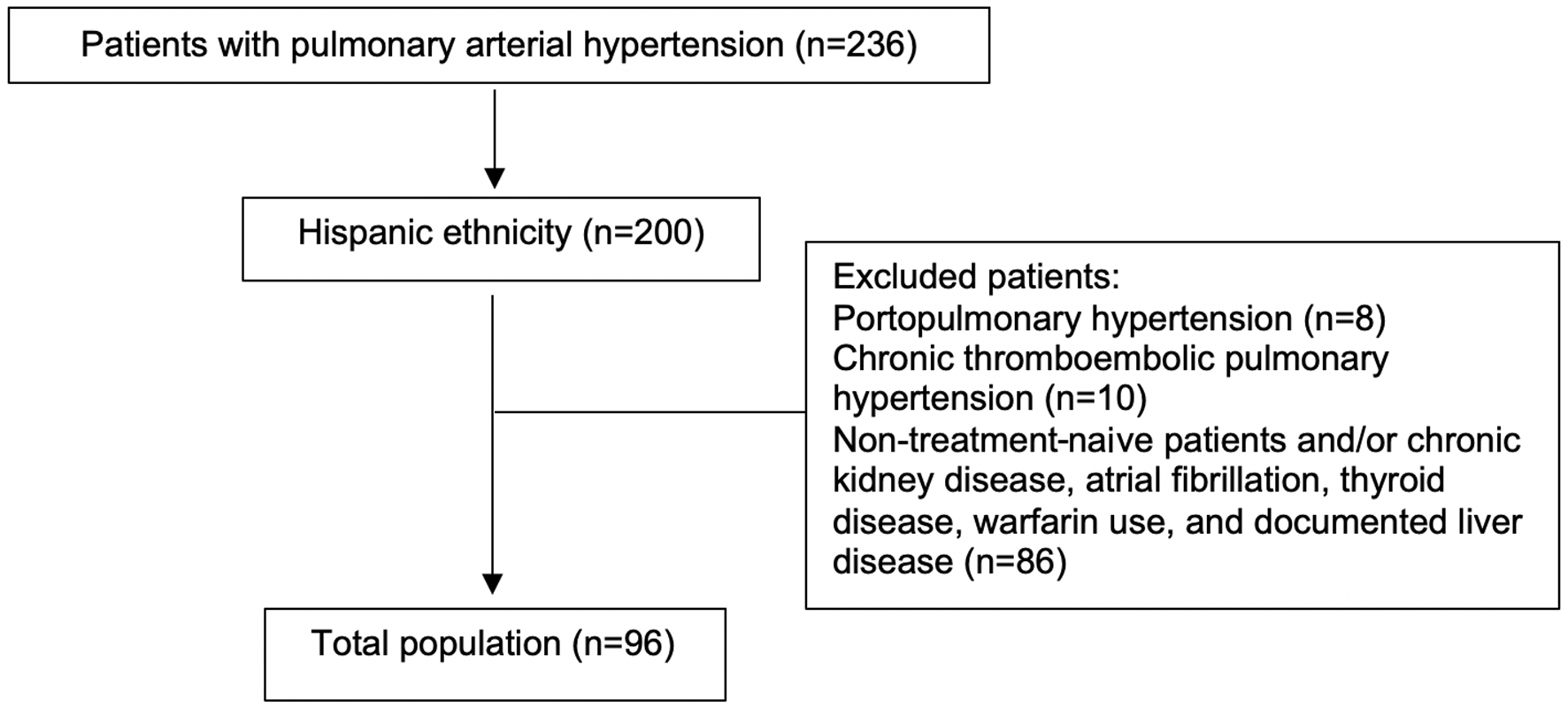 Click for large image | Figure 1. Flow diagram of patient selection. |
 Click to view | Table 1. Baseline Characteristics and NFS |
 Click to view | Table 2. Correlation of NFS With Baseline Variables |
Estimated median survival was significantly reduced in patients with advanced fibrosis scores (4.6 versus 3.0 years, P < 0.01). Cox regression analysis showed that NFSs were significantly associated with survival in a univariate model (hazard ratio (HR) 2.4, P = 0.001). Other significant non-invasive predictors of mortality at baseline were NT-proBNP (HR 1.1, P = 0.001), albumin (HR 2.1, P = 0.001), 6MWD (HR 1.1, P = 0.0023), and WHO-FC (HR 2.7, P = 0.001). After adjusting for age and gender, NFSs remained significant predictors of poor outcomes (HR 2.0, P = 0.031) (Table 3).
 Click to view | Table 3. Non-Invasive Risk Assessment Using Cox Regression Analysis |
Kaplan-Meier analysis showed that patients with advanced fibrosis scores had worse 3-year survival when compared with patients with low fibrosis scores (F0-F2) (Fig. 2, log-rank < 0.01).
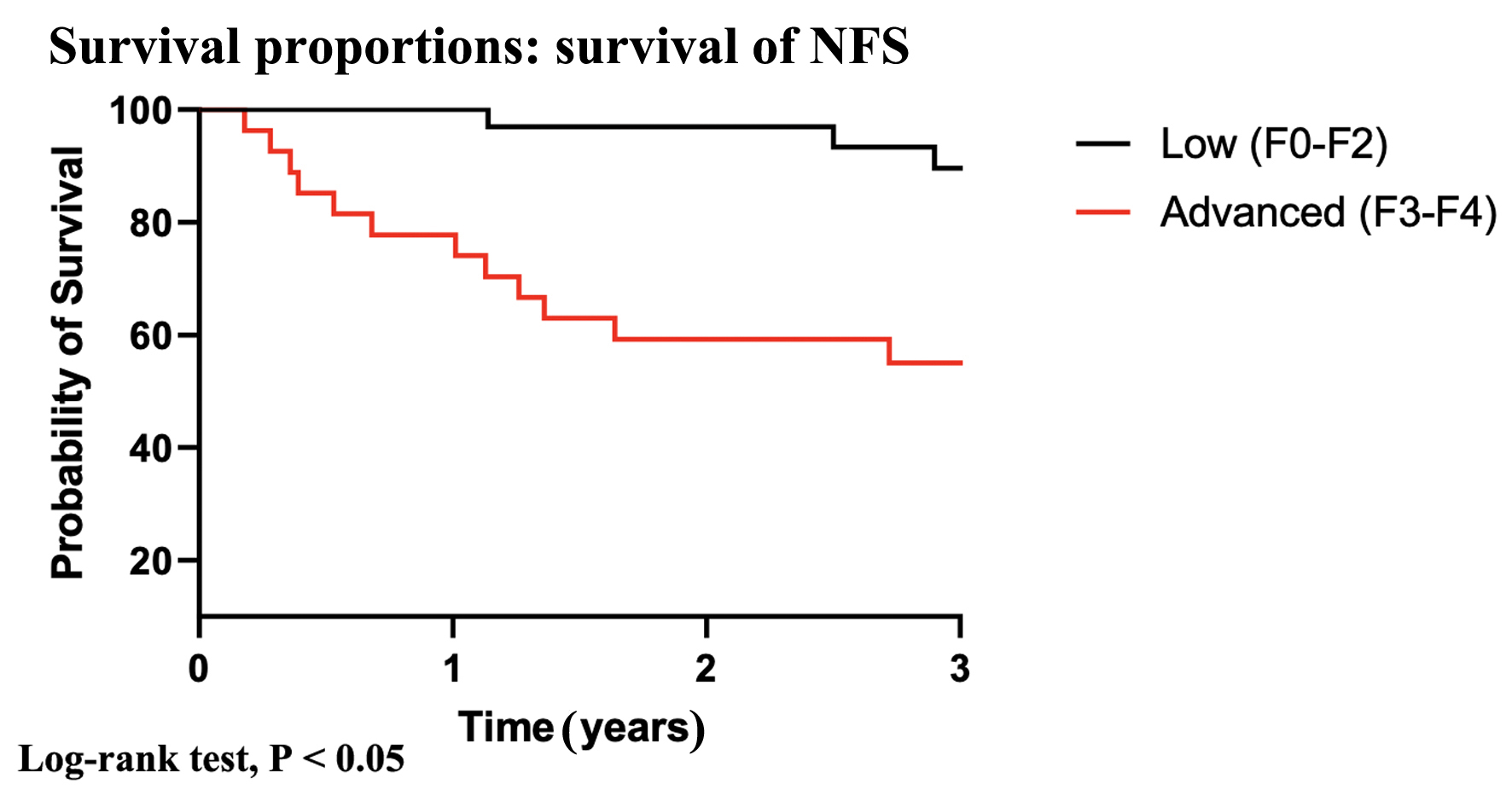 Click for large image | Figure 2. Kaplan-Meier analysis according to low and advanced fibrosis scores. |
| Discussion | ▴Top |
The lung-liver relationship is reciprocal, and liver disease clearly has implications on the anatomy and function of the pulmonary circulation, as seen in porto-pulmonary hypertension, hepato-pulmonary syndrome [5] and Fontan physiology, causing pulmonary arteriovenous malformations [6]. Vice versa, right heart strain has significant clinical implications on liver function and liver dysfunction is common in PAH patients [4]. The exact pathophysiology of liver dysfunction in PAH is poorly understood: hepatic congestion from right heart pressure overload and alteration in inflammatory, hormonal and genetic pathways have been implicated [4]. NAFLD is a clinicopathologic liver disease with a wide histological spectrum ranging from simple steatosis to cirrhosis and is associated with increased morbidity and mortality [8].
The NFS was initially created in 2007 as a non-invasive assessment for liver fibrosis in patients with NAFLD and was found to have a negative predictive value of advanced fibrosis (88-93%) when the lower cut-off was used (< -1.455), and a high positive predictive value of advanced fibrosis (82-90%) when the higher cut-off was used (> 0.675) [9]. NFSs were found to have a strong correlation with mortality, cardiovascular events, atheromatous plaque formation, and impaired renal function, signifying the correlation between NAFLD and metabolic syndrome [10, 11]. NFS has been validated as a non-invasive tool of assessing hepatic fibrosis with the use of routinely ordered labs.
To date, the incidence and prevalence of liver fibrosis in Hispanic patients with PAH is unknown. In our cohort 27% of patients had signs of advanced fibrosis based on NFSs. A retrospective analysis of ICD-10 codes in over 9,000 patients with PH found that 7% carried a diagnosis of NAFLD [12]. The prevalence of NAFLD in patients with chronic heart failure has been reported between 25% and 37% [13-15]. This indicates that NAFLD is likely an underreported and underrecognized comorbidity in heart failure patients, including patients with PAH. Our data support the notion that especially Hispanic PAH patients are at high risk for NAFLD. Despite analyzing a female predominant cohort, advanced fibrosis scores were more frequent in male patients. This is in line with other studies that found an increasing male prevalence with higher fibrosis scores [13]. In patients without evidence of heart failure, the NAFLD prevalence seems to be higher in men, when compared with premenopausal women; however, this relationship seems to be reversed when men are compared to postmenopausal women [16], implicating protective effects of female hormones on the liver. Our cohort had a median age of 49, it is therefore possible that premenopausal females had some protection against NAFLD development. Young females at childbearing age are at heightened risk for the development of PAH but seem to have a better overall prognosis when compared to male counterparts [17-20]. This conundrum has been termed the “estrogen paradox” in PAH. The survival in PAH is mainly determined by the ability of the RV to cope with increased afterload, and improved survival in females was linked to enhanced RV function, implicating that estrogens might have protective effects on the RV [21]. It is therefore possible that in PAH, female hormones protect the liver by advantageous hepatic fat metabolism [22] and by reduced congestion due to improved RV function.
Primary NAFLD has a strong association with the metabolic syndrome [8]. In patients with metabolic syndrome, insulin resistance and subsequent hyperinsulinemia are associated with altered glucose and lipid metabolism and subsequently, hepatic lipid accumulation leading to steatohepatitis [23, 24]. The metabolic syndrome is characterized by elevated very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), triglycerides (TGs), hepatic insulin resistance, low-grade systemic inflammation, and decreased liver insulin extraction [25-27]. In patients with advanced cirrhosis, disturbed hepatic blood flow has been linked to insulin resistance and hyperinsulinemia [28, 29]. In our cohort, patients with the highest NFSs had the highest prevalence of risk factors for metabolic syndrome, indicating that these risk factors contribute to liver pathology in patients with PAH. However, recent data implicate that even though PAH patients have many clinical features of the metabolic syndrome, significant differences exist. For instance, elevated serum TGs levels, peripheral and hepatic insulin resistance are not classic features of PAH metabolism [4]. In fact, congestive hepatopathy from right heart failure is associated with reduction in high-density lipoprotein (HDL) production and increased hepatic insulin clearance [30]. Therefore, patients with PAH and right heart failure have a different metabolic profile, compared to patients with “classic” metabolic syndrome, increased cardiovascular morbidity, and NAFLD [31]. The cause-and-effect relationship between liver fibrosis and cardiac dysfunction is a matter of ongoing research; however, our data suggest that in PAH, liver fibrosis is not only caused by diabetes, hyperlipidemia, obesity, or hypertension (Table 1). There is evidence that NAFLD increases the risk for heart failure [31]. Our study supports the notion that cardiac dysfunction plays a central role in the development of liver fibrosis. Right heart failure leads to increased pressure in the sublobular hepatic veins producing sinusoidal congestion, which may result in bridging fibrosis between adjacent central veins [32]. These histopathological findings are not only seen in right heart failure but have also been described in patients with diastolic dysfunction [33].
In our cohort, advanced NFSs were associated with decreased survival in univariate and multivariate analysis, when adjusted for established non-invasive risk predictors (NT-proBNP, WHO-FC, and 6MWD). Advanced NFSs were significantly related to age, male gender, worse WHO-FC, lower 6MWD, elevated right arterial pressures and NT-proBNP levels, indicating that NFSs assimilate several clinical, functional, hemodynamic and biochemical indicators of poor PAH outcomes. It is therefore interesting to note that NFSs added prognostic value to the established non-invasive risk parameters (Cox regression). It is unclear if liver fibrosis score is a modifiable risk factor in PAH. To date, there are no approved medical therapies for liver fibrosis. Many pharmaceutical and dietary interventions are currently investigated to treat liver fibrosis, but their role in PAH-associated hepatic dysfunction is unclear [34]. Anticoagulation to restore congested liver blood flow has been shown to have beneficial effects in patients with advanced cirrhosis [35]. The use of anticoagulants has also been implicated in improving survival in patients with idiopathic PAH [36, 37]. This is certainly an area that will require further investigation via clinical trials, since the decision to use anticoagulants in PAH needs to involve an individualized risk-benefit assessment. Other clinical considerations in patients with PAH and elevated NFSs are correction of risk factors for liver disease and reduction of right-sided filling pressures by optimizing PAH-targeted therapies and diuresis. Furthermore, special attention should be paid to nutrition counseling and weight loss in obese patients.
Limitations
Our study has several limitations, such as a relatively small cohort and its retrospective nature. Importantly, none of our patients had a liver biopsy to assess the degree of fibrosis. Our study lacks data on liver imaging (ultrasonography or computed tomography) that might be supportive of the NFSs. Furthermore, our study did not investigate if PAH-targeted therapies are associated with changes in NFSs.
Conclusion
Age and male gender in Hispanic patients with PAH are associated with higher risk for developing advanced liver fibrosis, which emerged as an independent risk factor for poor outcomes. Besides risk factors for metabolic syndrome, right heart strain seems to be an important contributor to liver fibrosis in PAH. Our study suggests that Hispanic patients with PAH and risk factors for metabolic syndrome should be screened for co-existing liver pathology by calculating NFSs. Further research is needed to better understand the complex relationship between the lungs and liver in PAH, and to investigate if reducing NFSs is a modifiable risk factor in PAH.
| Supplementary Material | ▴Top |
Suppl 1. ICD-10 codes with the corresponding disease.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was waived by our institution IRB.
Author Contributions
Concept and design of the work: MAK, NN, MZ, HA, DM, GG, YK, and HG. Analysis: NN. Drafting the paper: MAK and NN. Revising the paper: MAK, NN, MZ, HA, DM, GG, YK, and HG. Guarantor: NN.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
6MWD: 6-minute walk distance; mPAP: mean pulmonary arterial pressure; NAFLD: non-alcoholic fatty liver disease; NFS: non-alcoholic fatty liver disease fibrosis score; PAH: pulmonary arterial hypertension; PCWP: pulmonary capillary wedge pressure; PH: pulmonary hypertension; RV: right ventricle; TTUHSCEP: Texas Tech University Health Sciences Center El Paso; UMC: University Medical Center; WHO: World Health Organization; WHO-FC: World Health Organization functional class
| References | ▴Top |
- Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913.
doi pubmed pmc - Condon DF, Nickel NP, Anderson R, Mirza S, de Jesus Perez VA. The 6th World Symposium on Pulmonary Hypertension: what's old is new. F1000Res. 2019;8(F1000 Faculty Rev):888.
doi pubmed pmc - Nickel NP, Yuan K, Dorfmuller P, Provencher S, Lai YC, Bonnet S, Austin ED, et al. Beyond the lungs: systemic manifestations of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2020;201(2):148-157.
doi pubmed pmc - Nickel NP, Galura GM, Zuckerman MJ, Hakim MN, Alkhateeb H, Mukherjee D, Austin ED, et al. Liver abnormalities in pulmonary arterial hypertension. Pulm Circ. 2021;11(4):20458940211054304.
doi pubmed pmc - Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and hepatopulmonary syndrome. Lancet. 2004;363(9419):1461-1468.
doi pubmed - Kendall TJ, Stedman B, Hacking N, Haw M, Vettukattill JJ, Salmon AP, Cope R, et al. Hepatic fibrosis and cirrhosis in the Fontan circulation: a detailed morphological study. J Clin Pathol. 2008;61(4):504-508.
doi pubmed - Brunt EM. Pathology of nonalcoholic steatohepatitis. Hepatol Res. 2005;33(2):68-71.
doi pubmed - Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397(10290):2212-2224.
doi pubmed - Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846-854.
doi pubmed - Yu X, Chen C, Guo Y, Tong Y, Zhao Y, Wu L, Sun X, et al. High NAFLD fibrosis score in non-alcoholic fatty liver disease as a predictor of carotid plaque development: a retrospective cohort study based on regular health check-up data in China. Ann Med. 2021;53(1):1621-1631.
doi pubmed pmc - Hsieh MH, Wu KT, Chen YY, Yang JF, Lin WY, Chang NC, Lin CY, et al. Higher NAFLD fibrosis score is associated with impaired eGFR. J Formos Med Assoc. 2020;119(1 Pt 3):496-503.
doi pubmed - Jordens MS, Luedde M, Roderburg C, Demir M, Luedde T, Kostev K, Loosen SH. Pulmonary hypertension is associated with an increased incidence of NAFLD: A retrospective cohort study of 18,910 patients. J Intern Med. 2021;290(4):886-893.
doi pubmed - Peters AE, Pandey A, Ayers C, Wegermann K, McGarrah RW, Grodin JL, Abdelmalek MF, et al. Association of liver fibrosis risk scores with clinical outcomes in patients with heart failure with preserved ejection fraction: findings from TOPCAT. ESC Heart Fail. 2021;8(2):842-848.
doi pubmed pmc - Takahashi T, Watanabe T, Shishido T, Watanabe K, Sugai T, Toshima T, Kinoshita D, et al. The impact of non-alcoholic fatty liver disease fibrosis score on cardiac prognosis in patients with chronic heart failure. Heart Vessels. 2018;33(7):733-739.
doi pubmed - Yoshihisa A, Sato Y, Yokokawa T, Sato T, Suzuki S, Oikawa M, Kobayashi A, et al. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Fail. 2018;5(2):262-270.
doi pubmed pmc - Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, Abdelmalek MF, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70(4):1457-1469.
doi pubmed pmc - Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122(2):164-172.
doi pubmed - Hoeper MM, Huscher D, Ghofrani HA, Delcroix M, Distler O, Schweiger C, Grunig E, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol. 2013;168(2):871-880.
doi pubmed - Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122(2):156-163.
doi pubmed - Shapiro S, Traiger GL, Turner M, McGoon MD, Wason P, Barst RJ. Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest. 2012;141(2):363-373.
doi pubmed - Jacobs W, van de Veerdonk MC, Trip P, de Man F, Heymans MW, Marcus JT, Kawut SM, et al. The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest. 2014;145(6):1230-1236.
doi pubmed pmc - Lee C, Kim J, Jung Y. Potential therapeutic application of estrogen in gender disparity of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Cells. 2019;8(10):1259.
doi pubmed pmc - Angulo P. Nonalcoholic fatty liver disease and liver transplantation. Liver Transpl. 2006;12(4):523-534.
doi pubmed - Angulo P. Nonalcoholic fatty liver disease. Rev Gastroenterol Mex. 2005;70(Suppl 3):52-56.
pubmed - Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162.
doi pubmed pmc - Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17(5):644-656.
doi pubmed pmc - Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. 2009;42(13-14):1331-1346.
doi pubmed - Maruyama H, Kobayashi K, Kiyono S, Yokosuka O. Interrelationship between insulin resistance and portal haemodynamic abnormality in cirrhosis. Int J Med Sci. 2017;14(3):240-245.
doi pubmed pmc - Ishikawa T, Shiratsuki S, Matsuda T, Iwamoto T, Takami T, Uchida K, Terai S, et al. Occlusion of portosystemic shunts improves hyperinsulinemia due to insulin resistance in cirrhotic patients with portal hypertension. J Gastroenterol. 2014;49(9):1333-1341.
doi pubmed - Melenovsky V, Benes J, Franekova J, Kovar J, Borlaug BA, Segetova M, Tura A, et al. Glucose homeostasis, pancreatic endocrine function, and outcomes in advanced heart failure. J Am Heart Assoc. 2017;6(8):e005290.
doi pubmed pmc - Wijarnpreecha K, Lou S, Panjawatanan P, Cheungpasitporn W, Pungpapong S, Lukens FJ, Ungprasert P. Association between diastolic cardiac dysfunction and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Dig Liver Dis. 2018;50(11):1166-1175.
doi pubmed - Bayraktar UD, Seren S, Bayraktar Y. Hepatic venous outflow obstruction: three similar syndromes. World J Gastroenterol. 2007;13(13):1912-1927.
doi pubmed pmc - Louie CY, Pham MX, Daugherty TJ, Kambham N, Higgins JP. The liver in heart failure: a biopsy and explant series of the histopathologic and laboratory findings with a particular focus on pre-cardiac transplant evaluation. Mod Pathol. 2015;28(7):932-943.
doi pubmed - Weiskirchen R, Weiskirchen S, Tacke F. Recent advances in understanding liver fibrosis: bridging basic science and individualized treatment concepts. F1000Res. 2018;7(F1000 Faculty Rev):921.
doi pubmed pmc - Villa E, Camma C, Marietta M, Luongo M, Critelli R, Colopi S, Tata C, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143(5):1253-1260.e1254.
doi pubmed - Khan MS, Usman MS, Siddiqi TJ, Khan SU, Murad MH, Mookadam F, Figueredo VM, et al. Is anticoagulation beneficial in pulmonary arterial hypertension? Circ Cardiovasc Qual Outcomes. 2018;11(9):e004757.
doi pubmed pmc - Olsson KM, Delcroix M, Ghofrani HA, Tiede H, Huscher D, Speich R, Grunig E, et al. Anticoagulation and survival in pulmonary arterial hypertension: results from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA). Circulation. 2014;129(1):57-65.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


