| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 14, Number 6, December 2023, pages 437-445
Is the Blood Pressure-Enabled Smartwatch Ready to Drive Precision Medicine? Supporting Findings From a Validation Study
Wan Ling Leea, d , Mahmoud Danaeeb, Adina Abdullahc, Li Ping Wongb
aDepartment of Nursing Science, Faculty of Medicine, Universiti Malaya, 50603 Kuala Lumpur, Malaysia
bDepartment of Social and Preventive Medicine, Faculty of Medicine, Universiti Malaya, 50603 Kuala Lumpur, Malaysia
cDepartment of Primary Care Medicine, Faculty of Medicine, Universiti Malaya, 50603 Kuala Lumpur, Malaysia
dCorresponding Author: Wan Ling Lee, Department of Nursing Science, Faculty of Medicine, Universiti Malaya, 50603 Kuala Lumpur, Malaysia
Manuscript submitted September 1, 2023, accepted November 8, 2023, published online December 9, 2023
Short title: BP-Enabled Smartwatch to Drive Precision Medicine
doi: https://doi.org/10.14740/cr1569
| Abstract | ▴Top |
Background: The popular wrist-worn wearables recording a variety of health metrics such as blood pressure (BP) in real time could play a potential role to advance precision medicine, but these devices are often insufficiently validated for their performance to enhance confidence in its use across diverse populations. The accuracy of BP-enabled smartwatch is assessed among the multi-ethnic Malaysians, and findings is discussed in comparison with conventional automated upper-arm BP device.
Methods: Validation procedures followed the guidelines by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Universal Standard (ISO 81060-2:2018). Quota sampling was employed to obtain eligible patients with normal and abnormal BP as per guideline. The measurements of BP were taken at wrist using HUAWEI WATCH D (test BP); and the readings were assessed against reference BP by the mercury sphygmomanometer. Agreement statistics and linear regression analyses were performed.
Results: BP measurements (234 data pairs) from 78 patients that fulfilled AAMI/ESH/ISO protocol were analyzed. The BP readings taken by the HUAWEI WATCH D were comparable to reference BP by sphygmomanometer based on 1) Criterion 1: systolic blood pressure (SBP) = -0.034 (SD 5.24) and diastolic blood pressure (DBP) = -0.65 (SD 4.66) mm Hg; and 2) Criterion 2: SBPs = -0.034 (SD 4.18) and DBPs = -0.65 (SD 3.94) mm Hg. Factors of sociodemographic characteristics, anthropometric measurements, cardiovascular comorbidities, and wrist hair density were not significantly associated with the mean BP differences.
Conclusions: HUAWEI WATCH D fulfilled criteria 1 and 2 of the AAMI/ESH/ISO Universal Standard (ISO 81060-2:2018) guidelines. It can be recommended for clinical use across a wider population. The rich data from real-time BP measurements in concurrent with other health-related parameters recorded by the smartwatch wearable offer opportunities to drive precision medicine in tackling therapeutic inertia by personalizing BP control regimen.
Keywords: mHealth; Wearables; Smartwatch; Blood pressure; Validation; Precision medicine; Precision health
| Introduction | ▴Top |
High blood pressure (BP) or hypertension and pre-hypertension are significant risk factors that contribute to cardiovascular-related deaths and disabilities [1, 2]. The global disease burden of hypertension is huge with an estimated 1.28 billion adults aged 30 - 79 years having high BP, but only half of them is aware of their BP; and two-thirds of them live in low-to-middle-income countries [3]. To tackle the persistent issue of suboptimal detection, treatment and control of hypertension, more clinicians and scientists are showing greater interest to explore the potential use of mobile-health (mHealth) technologies to enhance out-of-office BP monitoring. The greater emphasis on out-of-office BP measurements as an essential component of BP control is clearly advocated in the recent guidelines [4]. The advancing wearable technology, particularly the wrist-worn devices, can address the limitations of office BP measurement that could overlook specific BP phenotypes such as the white coat and masked hypertension [5]. The accuracy of BP measurement by wrist-worn BP devices is crucial to detect elevated or problematic BP readings and to guide the decision-making in response to the BP measurements.
Despite the fact that several wrist-worn BP devices are already available in the commercial market, too few studies have been devoted to investigating the accuracy of the devices across different population types and countries. A review of BP measurement using photoplethysmography (PPG), transdermal optical imaging, phonocardiography and seismocardiography signals can be affected by factors of gender differences (e.g., in skin thickness, heart rate, blood vessel diameter), comorbidities, abnormal BP range, skin tone, and aging [6]. A higher body mass index and darker skin tone [7, 8] and dense hair follicle [9] induce a relative loss of PPG signal. Pulse pressure and the shape of the artery-cuff pressure/volume curve affecting oscillometer readings of BP are influenced by factors related to gender, ageing, comorbidities and others. Measurements over clothing is a physical barrier that affects accuracy of readings by automated oscillometric upper-arm BP monitor [10].
Among the several wrist-worn BP devices available in the market is the HUAWEI WATCH D developed by the HUAWEI Technologies Co. Ltd. (Shenzhen, China). Additionally, the HUAWEI WATCH D is a smartwatch equipped with other functions (e.g., heart rate, stress, sleep, oxygen saturation, etc.), and it has accompanying smartphone app called Huawei Health. To date, the HUAWEI WATCH D has been validated only among the Chinese population in China [11, 12]. These studies were carried out in accordance with guidelines stipulated by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Universal Standard (ISO 81060-2:2018). We aim to validate the accuracy of BPs measured by HUAWEI WATCH D among the multi-ethnic and heterogenous population of Malaysia by adopting similar protocols by Zhang et al [11] and Wang et al [12]. Cross-population validation of devices is essential to enhance confidence in their use among diverse characteristics of people.
| Materials and Methods | ▴Top |
The procedural steps were carried out by the same observers comprised of a medical and a nurse graduate and supervised by a senior nurse in consultation with an expert physician. Observers were trained in the laboratory to take systolic blood pressure (SBP) and diastolic blood pressure (DBP) measurements by auscultation method. A pilot study on 10 patients was conducted to familiarize them with the study protocol and test device. The data collection and analysis procedural were performed according to guidelines by AAMI/ESH/ISO Universal Standard (ISO 81060-2:2018) [13-15].
Study settings and participants
This study was conducted at a large university medical center in Kuala Lumpur, Malaysia between November and December 2022. Adult patients who visited the primary care clinic and who were admitted to the general medical-surgical adult wards were recruited if they met the inclusion criteria: aged ≥ 18 years, cognitively alert and able to cooperate with researchers’ instructions. Study exclusion criteria were pregnancy, aged above 70 years due to the differences in vascular compliance, arrhythmia due to inaudible phase V Korotkoff sounds to determine the DBP, serious orthopedic problems of upper arms (e.g., fractures) and unstable vital signs. Quota sampling was employed to achieve targeted minimum (min) sample distribution by gender (i.e., min 30% males, min 30% females) across wide-ranging BP readings (i.e., min 5% SBP < 100 mm Hg, min 5% DBP ≤ 60 mm Hg; min 5% SBP ≥ 160 mm Hg; min 5% DBP ≥ 100 mm Hg; min 20% SBP ≥ 140 mm Hg; min DBP ≥ 85 mm Hg).
Test device
The HUAWEI WATCH D measures BP based on the oscillometry method involving using a micro-pump and a detachable bladder cuff. During cuff inflation at the wrist, the detected pulse wave was analyzed using an algorithm for determining the SBP and DBP. The watch has an airline design that is based on engineering fluid simulation and the airbag is ergonomically arched to ensure a comfortable fit on the wrist when it is inflated. The measurement range of the HUAWEI WATCH D is set at 60 - 230 mm Hg for SBP and 40 - 160 mm Hg for DBP. Further details of device features are available elsewhere [11, 12].
We prepared one watch attached with a medium and a large cuff for wrist circumference of 130 - 160 mm and 161 - 200 mm, respectively. As per the manufacturer’s instruction, we measured the participant’s wrist circumference before applying the watch with a cuff size appropriate for the individual. To maintain the device position at the same level as the heart, the left arm with the wrist-worn device was kept bent with its palm in a naturally straight position and was placed on the chest; the left arm was kept supported at the elbow by the right hand.
Reference BP
The manual BP measurement was taken independently and simultaneously by two observers using the same double-headed stethoscope (Y-tube) and a calibrated mercury sphygmomanometer. The arm cuffs covered 75-100% of the upper arm circumference, and the width covers 37-50% of the arm circumference. The SBP reading was determined based on the audible phase I Korotkoff sounds, and the DBP reading based disappearance of the Korotkoff sounds at phase V.
Validation procedures
Before the test, information was gathered from the medical record and from patients when needed to obtain data on socio-demography (i.e., age, gender, ethnicity, education, occupation, household income), medical illness (including cardiovascular and cerebrovascular diseases, and hypertension diagnosis), weight and height. A standard set of colored photographs depicting different hair density of forearm and wrist was used by two observers to record their ratings, independently based on 4-point scale, i.e., I (“nil”), II (“sparse”), III (“moderate”) and IV (“dense”) [16].
After participants emptied their bladders, they were kept rested and relaxed in calm surroundings for about 30 min before validation procedure. We used the same-arm sequential BP measurement approach. The watch and sphygmomanometer cuff were continuously worn on the left wrist and left upper arm, respectively, while the BP measurements were obtained at heart level in alternating sequence between the two devices. During BP measurement, participants sat comfortably on a chair with an upright backrest and remained quiet with their legs uncrossed.
After obtaining two entry BP measurements (reference R0 and test device T0), we proceeded to collect validation data which consisted of four reference BP measurements (R1 to R4) by mercury sphygmomanometer alternating with three test BP measurements (T1 to T3) by the watch device in the following sequence R1-T1-R2-T2-R3-T3-R4. Each reference BP (i.e., R1 to R4) was the averaged of two simultaneous BP readings reported by observers. The time between each set of BP measurements was at least 60 s and procedural duration kept within 30 min per participant.
Statistical analysis
Data analysis was performed according to the AAMI/ESH/ISO Universal Standard guidelines. For criterion 1 (individual BP readings evaluation) of AAMI/ESH/ISO Universal Standard, each test BP was compared against the average of the previous and next reference BP reading (i.e., T1 versus the average of R1-R2, T2 versus average of R2-R3, T3 versus average of R3-R4). Each participant yielded three values of mean BP differences (test BP-reference BP). The mean and respective standard deviation (SD) of the differences were calculated to fulfill the requirement of criterion 1 for a mean BP difference of ≤ 5 mm Hg, and an SD of ≤ 8 mm Hg for SBP and DBP.
For criterion 2 (individual subject evaluation), a difference was defined as the mean of the three test SBPs or DBPs, as measured by the HUAWEI WATCH D minus the mean values of the three reference SBPs or DBPs. The SDs of the pairs of BP differences must be within the threshold defined by the mean test-reference BP difference listed in the AAMI/ESH/ISO Universal Standard (i.e., standard error of mean SBP/DBP ≤ 6.95/≤ 6.91).
Bland-Altman scatterplots provide visualization of data in assessing the test-reference BP differences. Linear regression analysis, adjusted for sociodemographic characteristics, anthropometric measurements and comorbidities, was conducted to investigate the association between the mean differences between the test and reference BP measurements by wrist hair follicles density. The SPSS, IBM Corp., Armonk, NY, USA (version 25) and JASP version 0.16.4.0 were used, and levels of significance were set at α of 0.05.
Ethical considerations
After ethical approval by the Institutional Review Board (UMMC-MREC No 2022729-11428), the study procedures were conducted in accordance with the Declaration of Helsinki and Caldicott principles. Prior to data collection, written informed consent was obtained from all participants after explaining the study using patient information sheet. Data security and participants’ confidentiality were maintained at all levels of data management.
| Results | ▴Top |
A total of 116 participants were recruited, and 38 cases were excluded solely based on reference BP discrepancy criteria (and not test BPs) during data screening and cleaning [12]. As per AAMI/ESH/ISO guidelines [14], cases were excluded as follows: 1) Any two observers’ readings differed by > 4 mm Hg (either reference SBP or DBP); and 2) Any of the two adjacent reference BP readings (i.e., R1-R2 or R2-R3 or R3-R4) differed by > 12 mm Hg for SBP, or by > 8 mm Hg for DBP. The latter is known as “reference BP variation”, which reflects the continuous dynamic nature of BP, in which the beat-to-beat BP can vary markedly (from -24 mm Hg to 33 mm Hg) in response to usual extrinsic and behavioral factors [17]. Unlike healthy populations, patients are more likely to feel tired or anxious during the 30-min validation procedure.
A final of 234 data pairs (78 participants × 3 data pairs) was included in validation analysis. Participants’ distribution of sex (52.6% men, 47.4% women) and reference BPs met the sampling quota criterion. The percentages of high (≥ 160 mm Hg), medium (≥ 140 mm Hg), and low (≤ 100 mm Hg) SBPs were 5.1% (met the 5% criterion), 25.6% (met 20% criterion), and 11.5% (met 5% criterion), respectively. The percentages of high (≥ 100 mm Hg), medium (≥ 85 mm Hg), and low (≤ 60 mm Hg) DBPs were 6.4% (met the 5% criterion), 24.3% (met 20% criterion), and 6.4% (met 5% criterion), respectively.
Table 1 shows the characteristics of 78 participants. Sample distribution of the Malays (46.2%), Chinese (25.6%) and Indians (24.4%) were representative of the major ethnic populations in Peninsula Malaysia. The most common comorbidities were hypertension (47.4%), diabetes mellitus (28.2%) and ischemia heart disease (25.6%). Participants’ mean age was 50.06 (SD: 15.19 years; range: 18 - 70 years), mean body mass index was 26.11 (SD: 5.78), and mean wrist circumference was 17.36 mm (SD: 1.68).
 Click to view | Table 1. Descriptive Statistics for Sociodemographic, Anthropometric and Medical Characteristics (N = 78) |
Table 2 shows that HUAWEI WATCH D passed the validation requirement. The mean BP differences were -0.034 (SD 5.24) mm Hg for SBP and -0.65 (SD 4.66) mm Hg for DBP, in accordance with criterion 1. The standard error of mean differences between the test-watch and reference BPs were 4.18 mm Hg for SBP and 3.94 mm Hg for DBP, in accordance with criterion 2.
 Click to view | Table 2. Validation Results Based on Criterion 1 and Criterion 2 |
Figure 1 illustrates a graphical representation of the Bland-Altman analysis showing an average of bias at 0.034 with limits of agreement ranging from -8.148 to 8.216 mm Hg for SBP and an average of bias of 0.649 with limits of agreement from -7.065 to 8.362 for DBP. The results indicated that the estimated bias for both SBP and DBP were not significantly different from 0.
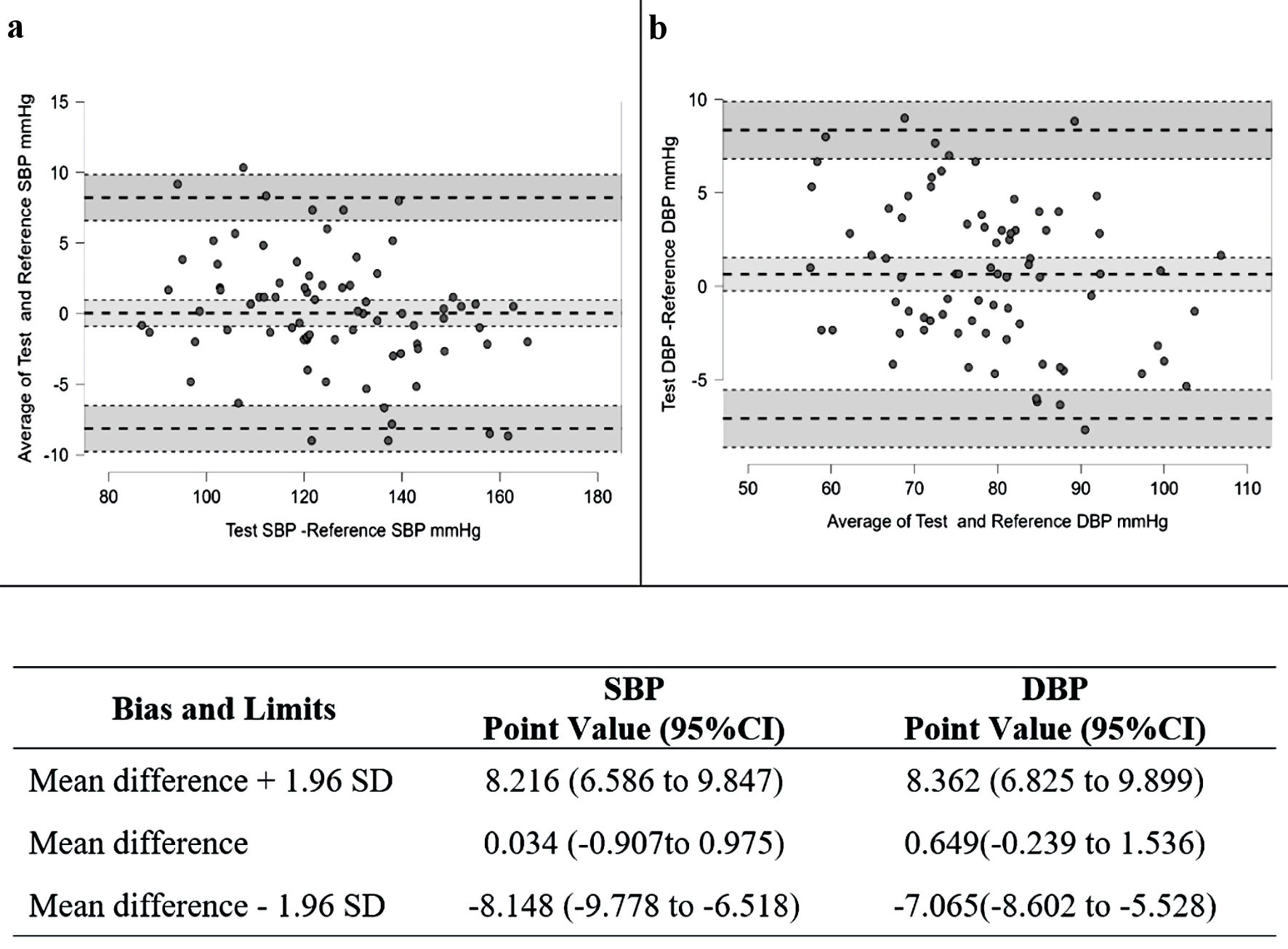 Click for large image | Figure 1. Bland-Altman scatter plots and results of the mean differences between watch device and the reference BPs for the systolic blood pressure (SBP) (a) and diastolic blood pressure (DBP) (b). SD: standard deviation; CI: confidence interval. |
A comparison among three ethnic groups (i.e., Malay, Chinese and Indian) was done using one-way analysis of variance (ANOVA) (Table 3) and Bland-Altman scatterplots (Fig. 2) for both systolic and diastolic measurement accuracy; and the results indicated that there were no significant differences among groups.
 Click to view | Table 3. Results of the Mean Differences of SBP and DBP Between Watch Device and the Reference BPs by Malay, Chinese and Indian Ethnic Groups |
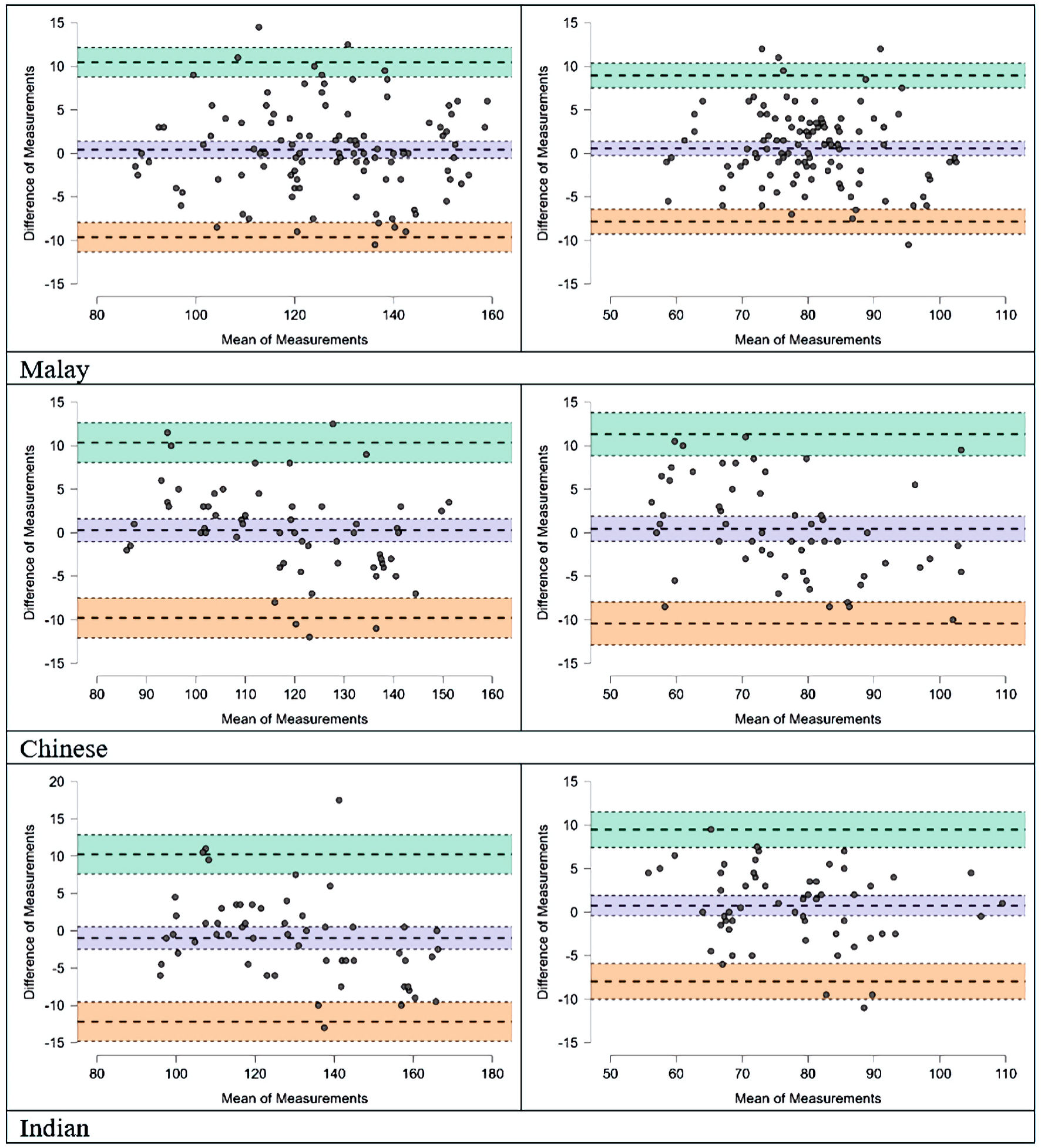 Click for large image | Figure 2. Bland-Altman scatter plots on mean difference between watch device and the reference BPs for the SBP (a) and DBP (b) by Malay, Chinese and Indian ethnic groups. |
Additional subgrouping analysis shows no significant difference in mean SBP difference and DBP difference by sex, education, income, employment and comorbidities groupings, and no significant correlation with age, wrist circumference and body mass index. In linear regression analysis adjusted for sociodemographic characteristics, anthropometric measurements and comorbidities, we found no significant associations in the mean differences between the test and reference by skin hair density (SBP: β = -0.157, P = 0.184; DBP: β = -0.208, P = 0.068).
| Discussion | ▴Top |
Study findings show the device, i.e., HUAWEI WATCH D fulfills criteria 1 and 2 of the AAMI/ESH/ISO Universal Standard (ISO 81060-2:2018) guidelines. There is an adequate agreement in measurements by the BP-enabled smartwatch and mercury sphygmomanometer as shown by the graphical presentations of the test-reference differences in the SBP and DBP. Additional analysis on criterion 1 did not demonstrate statistical significance (P values > 0.05) in the mean BP differences by factors of ethnicity and other socio-demography, as well as body mass index, wrist circumferences, and wrist hair density. Study findings support the use of the HUAWEI WATCH D among multi-ethnic Malaysians, and potentially across Southeast Asians subpopulation across the world. To date, there is no validation data available from populations of Europeans or Western countries to compare this study findings.
In reference to criterion 1, the mean differences between test and reference SBP/DPB of -0.034/-0.65 mm Hg observed in this study were slightly smaller than -0.25/-1.33 mm Hg reported by Wang et al [12], and -1.4/-0.2 mm Hg by Zhang et al [11], who validated the same device model on the general population of China. The HUAWEI WATCH D is comparable to two other similar cuff-based models such as the Omron HEM-6410T-ZM and Omron HEM-6410TZL with mean differences of SBP/DBP at -0.9/-1.1 mm Hg and 2.4/0.3 mm Hg, respectively [18]. These cuff-based wristwatch devices recorded a slightly smaller mean difference than the cuff-less model such as InBodyWATCH, which reported mean difference of SBP/DBP at 2.2/-0.2 mm Hg [19]. Notably, the mean BP difference by HUAWEI WATCH D is comparable to automated oscillometric, upper-arm BP monitor such as QMon-20 oscillometric with mean difference of SBP/DBP at 0.8/-0.5 mm Hg [20]. Therefore, the BP-enabled smartwatch can be a convenient alternative to out-of-office BP monitoring.
Implications to advancing precision medicine
Study findings recommending a validated BP-enabled smartwatch, such as HUAWEI WATCH D, for clinical use pose a significant implication to patient management and public health. Wristwatch enables unobtrusive BP-taking, user convenience and easier portability than automated arm devices. For example, HUAWEI WATCH D has user instructions displayed on its screen, and it can automatically detect hand posture to guide proper hand placing during the measurement process. These advantageous features of the wristwatch devices could further facilitate out-of-office BP measurement in a timely frequency. According to meta-analysis findings, out-of-office BP monitoring is one of the key strategies to tackle therapeutic inertia, which refers to failing in adjusting medication regimen when BP falls out of targeted range [21, 22]. Additionally, a validated wristwatch BP device could facilitate detection of different BP patterns that have prognostic relevance (e.g., nocturnal dipping, morning rise) and pathological BP variability [5].
Of note, the short-term BP variability that occurs within 24 h which includes minute-to-minute, hourly, and circadian changes have rendered all current methods of BP measurements have imperfect reproducibility. Notwithstanding the continuous dynamic nature of BP, the watch device has clinical utility by recording a range of readings to gauge BP control of an individual. Furthermore, the variation of BP readings captured by watch device provide rich data that are needed to support current research interest studying impact of BP variability on cardiovascular outcomes [17].
The collection of real-time BP measurements by wristwatch device concomitantly accompanied by other health-related parameters such as heart rate, oxygen saturation, physical activity, emotion, etc. can supply rich real-world data to drive precision medicine for personalized patient care that could address the issue of suboptimal BP control. In Malaysia, for example, only 45% of the 6.4 million hypertensive individuals attained good BP control [23]. The wristwatch BP devices enabling users to perform self-monitoring, and those who interact frequently with the watch-type devices are likely to be more motivated to comply with treatment even in a resource-limited community-based setting [24]; thus, devices with attractive interactive features are sought after, provided it met the user’s perception of good-value-for-money. To have a wider impact on population health, the validated device needs to be affordable to the masses who are mainly of the lower-to-middle income bracket.
On the other hand, a review of qualitative studies has found that self-monitoring could inflict psychological burden by triggering confusion and stress, such as anxiety over “bad” readings, a constant reminder of illness identity, uncertainties of interpreting and responding to out-of-target readings, concerns of unreliability and frequency of measures, etc. [25]. For optimized benefits, BP self-monitoring needs to be accompanied by intensive support such as counselling, education, behavioral management, medication management with decision, adherence contracts, feedbacks, and other co-interventions in order to achieve better BP control [5, 22, 26]. Wristwatch devices that allow telemonitoring by transmitting BP readings to clinicians are projected to show greater BP control for an extended period such as up to 5 years [27]. The role of wristwatch BP devices in preventive medicine among the general population is promising as it enables regular self-monitoring to detect and manage daily stress-induced BP elevation, especially among working adults [28].
Strengths and limitations
This study has the advantage of having samples that were proportionally representative of a multi-ethnic Malaysian population. Compared to previous studies [11, 12], wider possible factors of mean BP differences were tested in this study. The issue of optimal sample size of 33 versus 85 for validation of BP devices has been contentious [15], and there is inadequate empirical evidence to substantiate the minimum samples needed. We did a post-hoc power analysis using GPower (version 3.1.9.4) on the observed correlation between the test and reference BP of 0.92, and the results showed that the 78 samples had generated adequate power (1-β) of 0.99 at α level of 0.05. Hence, we view that adding a mere seven samples is futile as it would not significantly alter the study conclusion. After all, the oscillometric wristwatch BP devices that pass the validation criteria are still subjected to wide BP variations resulting from factors affecting hydrostatic pressure such as body movement, body and palm positioning, wrist placement to the heart level which narrows its use to non-ambulatory setting. This study could only substantiate accuracy of wristwatch BP at rest, hence, its accuracy during body movement is yet to be determined in future research.
Conclusions
The HUAWEI WATCH D fulfilled criteria 1 and 2 of the AAMI/ESH/ISO Universal Standard (ISO 81060-2:2018) guidelines when used in a sitting position with wrist at heart level. For proper BP measurements, users are required to follow the manufacturer’s instructions correctly. Given its acceptable accuracy and user convenience, the device can be recommended for clinical use; and by doing so, it will open more opportunities to drive precision medicine for personalized BP control.
Acknowledgments
The authors would like to thank all study participants for their willingness to take part in the study and Ng Bren Den for the administrative assistance.
Financial Disclosure
This work was supported by Huawei Technologies (Malaysia). The funders were not involved in study conception, data collection, analysis, and interpretation of data; in the writing of the manuscript; nor in the decision to submit the manuscript for publication.
Conflict of Interest
LWL received funding from Huawei Technologies (Malaysia).
Informed Consent
Written informed consent was obtained from all participants.
Author Contributions
LWL, AA and WLP were involved in the study conception. LWL worked on gaining ethical approval. LWL conducted the fieldwork in consultation with AA. MD performed data analysis and interpretation. LWL was the main writer of the draft with some input from AA, WLP and MD. All authors approved the final version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
AAMI/ESH/ISO: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization; BP: Blood pressure
| References | ▴Top |
- G. B. D. R. F. Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223-1249.
doi pubmed pmc - Qureshi AI, Suri MF, Kirmani JF, Divani AA, Mohammad Y. Is prehypertension a risk factor for cardiovascular diseases? Stroke. 2005;36(9):1859-1863.
doi pubmed - N. C. D. R. F. Collaboration. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398(10304):957-980.
doi pubmed pmc - Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104.
doi pubmed - Parati G, Lombardi C, Pengo M, Bilo G, Ochoa JE. Current challenges for hypertension management: From better hypertension diagnosis to improved patients' adherence and blood pressure control. Int J Cardiol. 2021;331:262-269.
doi pubmed - Frey L, Menon C, Elgendi M. Blood pressure measurement using only a smartphone. NPJ Digit Med. 2022;5(1):86.
doi pubmed pmc - Luo H, Yang D, Barszczyk A, Vempala N, Wei J, Wu SJ, Zheng PP, et al. Smartphone-based blood pressure measurement using transdermal optical imaging technology. Circ Cardiovasc Imaging. 2019;12(8):e008857.
doi pubmed - Ajmal, Boonya-Ananta T, Rodriguez AJ, Du Le VN, Ramella-Roman JC. Monte Carlo analysis of optical heart rate sensors in commercial wearables: the effect of skin tone and obesity on the photoplethysmography (PPG) signal. Biomed Opt Express. 2021;12(12):7445-7457.
doi pubmed pmc - Cosoli G, Spinsante S, Scalise L. Wrist-worn and chest-strap wearable devices: Systematic review on accuracy and metrological characteristics. Measurement. 2020;159:107789.
- Ozone S, Shaku F, Sato M, Takayashiki A, Tsutsumi M, Maeno T. Comparison of blood pressure measurements on the bare arm, over a sleeve and over a rolled-up sleeve in the elderly. Fam Pract. 2016;33(5):517-522.
doi pubmed - Zhang W, Zhou YN, Zhou Y, Wang JG. Validation of the watch-type HUAWEI WATCH D oscillometric wrist blood pressure monitor in adult Chinese. Blood Press Monit. 2022;27(5):353-356.
doi pubmed - Wang L, Xian H, Guo J, Li W, Wang J, Chen Q, Fu X, et al. A novel blood pressure monitoring technique by smart HUAWEI WATCH: A validation study according to the ANSI/AAMI/ISO 81060-2:2018 guidelines. Front Cardiovasc Med. 2022;9:923655.
doi pubmed pmc - Stergiou GS, Tzamouranis D, Protogerou A, Nasothimiou E, Kapralos C. Validation of the Microlife Watch BP Office professional device for office blood pressure measurement according to the International protocol. Blood Press Monit. 2008;13(5):299-303.
doi pubmed - Stergiou GS, Palatini P, Asmar R, Ioannidis JP, Kollias A, Lacy P, McManus RJ, et al. Recommendations and Practical Guidance for performing and reporting validation studies according to the Universal Standard for the validation of blood pressure measuring devices by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO). J Hypertens. 2019;37(3):459-466.
doi pubmed - Stergiou GS, Alpert B, Mieke S, Asmar R, Atkins N, Eckert S, Frick G, et al. A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. J Hypertens. 2018;36(3):472-478.
doi pubmed pmc - von Schuckmann LA, Hughes MC, Green AC, van der Pols JC. Forearm hair density and risk of keratinocyte cancers in Australian adults. Arch Dermatol Res. 2016;308(9):617-624.
doi pubmed - Kallioinen N, Hill A, Horswill MS, Ward HE, Watson MO. Sources of inaccuracy in the measurement of adult patients' resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017;35(3):421-441.
doi pubmed pmc - Kuwabara M, Harada K, Hishiki Y, Kario K. Validation of two watch-type wearable blood pressure monitors according to the ANSI/AAMI/ISO81060-2:2013 guidelines: Omron HEM-6410T-ZM and HEM-6410T-ZL. J Clin Hypertens (Greenwich). 2019;21(6):853-858.
doi pubmed pmc - Moon JH, Kang MK, Choi CE, Min J, Lee HY, Lim S. Validation of a wearable cuff-less wristwatch-type blood pressure monitoring device. Sci Rep. 2020;10(1):19015.
doi pubmed pmc - Rico-Martin S, Sanchez-Bacaicoa M, Calderon-Garcia JF, Labrador-Gomez PJ, De Nicolas Jimenez JM, Villa-Rincon J, Robles NR, et al. Validation of the QMon-20 oscillometric blood pressure monitor for professional office use in the general population according to the ANSI/ESH/ISO 81060-2:2018 protocol. Blood Press Monit. 2021;26(5):393-395.
doi pubmed - Agarwal R, Bills JE, Hecht TJ, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57(1):29-38.
doi pubmed - Tucker KL, Sheppard JP, Stevens R, Bosworth HB, Bove A, Bray EP, Earle K, et al. Self-monitoring of blood pressure in hypertension: A systematic review and individual patient data meta-analysis. PLoS Med. 2017;14(9):e1002389.
doi pubmed pmc - Institute for Public Health, National Health and Morbidity Survey (NHMS) 2019: Non-communicable diseases, healthcare demand, and health literacy - Key Findings. 2020. Ministry of Health Malaysia: Kuala Lumpur.
- Zhang Y, Yang N, Si G, Zhang Y, Dong Z, Huang Y, Tan X. What matters the adherence with BP 24-hr self-monitoring wearable device among hypertensive patients? A population-based survey. Transl Behav Med. 2020;10(4):1053-1063.
doi pubmed - Natale P, Ni JY, Martinez-Martin D, Kelly A, Chow CK, Thiagalingam A, Caillaud C, et al. Perspectives and experiences of self-monitoring of blood pressure among patients with hypertension: a systematic review of qualitative studies. Am J Hypertens. 2023;36(7):372-384.
doi pubmed pmc - Duan Y, Xie Z, Dong F, Wu Z, Lin Z, Sun N, Xu J. Effectiveness of home blood pressure telemonitoring: a systematic review and meta-analysis of randomised controlled studies. J Hum Hypertens. 2017;31(7):427-437.
doi pubmed - Bryant KB, Sheppard JP, Ruiz-Negron N, Kronish IM, Fontil V, King JB, Pletcher MJ, et al. Impact of self-monitoring of blood pressure on processes of hypertension care and long-term blood pressure control. J Am Heart Assoc. 2020;9(15):e016174.
doi pubmed pmc - Tomitani N, Kanegae H, Kario K. Self-monitoring of psychological stress-induced blood pressure in daily life using a wearable watch-type oscillometric device in working individuals with hypertension. Hypertens Res. 2022;45(10):1531-1537.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


