| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Review
Volume 14, Number 6, December 2023, pages 421-428
Mitral Annular Disjunction: Clinical Implications and Surgical Considerations
Ling Zhua, b, Yeow Leng Chuaa
aDepartment of Cardiothoracic Surgery, National Heart Centre Singapore, Singapore 169609, Singapore
bCorresponding Author: Ling Zhu, Department of Cardiothoracic Surgery, National Heart Centre Singapore, Singapore 169609, Singapore
Manuscript submitted October 4, 2023, accepted October 28, 2023, published online December 9, 2023
Short title: Mitral Annular Disjunction
doi: https://doi.org/10.14740/cr1584
- Abstract
- Introduction
- Diagnosis of MAD
- Clinical Implications of MAD
- Surgical Considerations in MAD
- Conclusions
- References
| Abstract | ▴Top |
Mitral annular disjunction is a cardiac structural abnormality characterized by the distinct separation between the top of the left ventricular myocardium and the mitral annulus supporting the posterior mitral leaflet occurring during systole. It has recently gained wide attention due to the increasing recognition of the link between mitral annular disjunction and arrhythmogenic mitral valve prolapse, particularly, with the increased risks of ventricular arrhythmias resulting in sudden cardiac death. This review has summarized the recent progress in the diagnostic modalities, clinical implications of mitral annular disjunction, and its specific surgical considerations.
Keywords: Mitral annular disjunction; Mitral valve prolapse; Mitral regurgitation; Ventricular arrhythmias; Mitral valve surgery
| Introduction | ▴Top |
Mitral annular disjunction (MAD) is a cardiac structural abnormality of the mitral valve (MV) characterized by the distinct separation between the left atrial (LA) wall-MV junction (mitral annulus) and the top of the left ventricular (LV) free wall. It was described as early as 1980s by Hutchins et al [1], when heart autopsies were performed for 25 cases of mitral valve prolapse (MVP). MAD was identified as high as 92%, although little attention was paid by then as MAD was deemed with no significant clinical consequence. Till 2005, Eriksson et al reported that the recognition of MAD prior to MV repair [2], with modification of the surgical techniques to correct the annular disjunction, were essential to achieve durable long-term results. Subsequently, MAD started to gain more clinical interest as it became more frequently recognized by echocardiography. It was found by Carmo et al [3] that the displacement of the mitral annulus has substantially impaired the mitral annular function, and the degree of the disjunction was correlated with the incidence of non-sustained ventricular tachycardia (NSVT).
To date, MAD has been widely reported to be associated with MVP, mitral regurgitation (MR) and cardiac arrhythmia, although the pathophysiology of MAD is yet fully understood. This review will summarize the recent progress in the modalities of diagnosis for MAD, the implications of MAD in different clinical entities, and the surgical considerations in managing MAD.
| Diagnosis of MAD | ▴Top |
MAD is predominantly found in the posterior mitral leaflet (particularly the P1 and P2 segments), where the mitral annulus is in close relationship with the LV free wall. When the posterolateral LV wall contracts during systole, the mitral annulus slides and “detaches” from the LV myocardium, then MAD occurs [4]. MAD can be diagnosed by various imaging modalities.
In transthoracic echocardiogram (TTE), MAD can be assessed in parasternal long axis view at the end of systole. The distance of disjunction can be measured by from the insertion point of the posterior leaflet on LA wall to the junction between the LV and LA myocardium, as demonstrated in Figure 1.
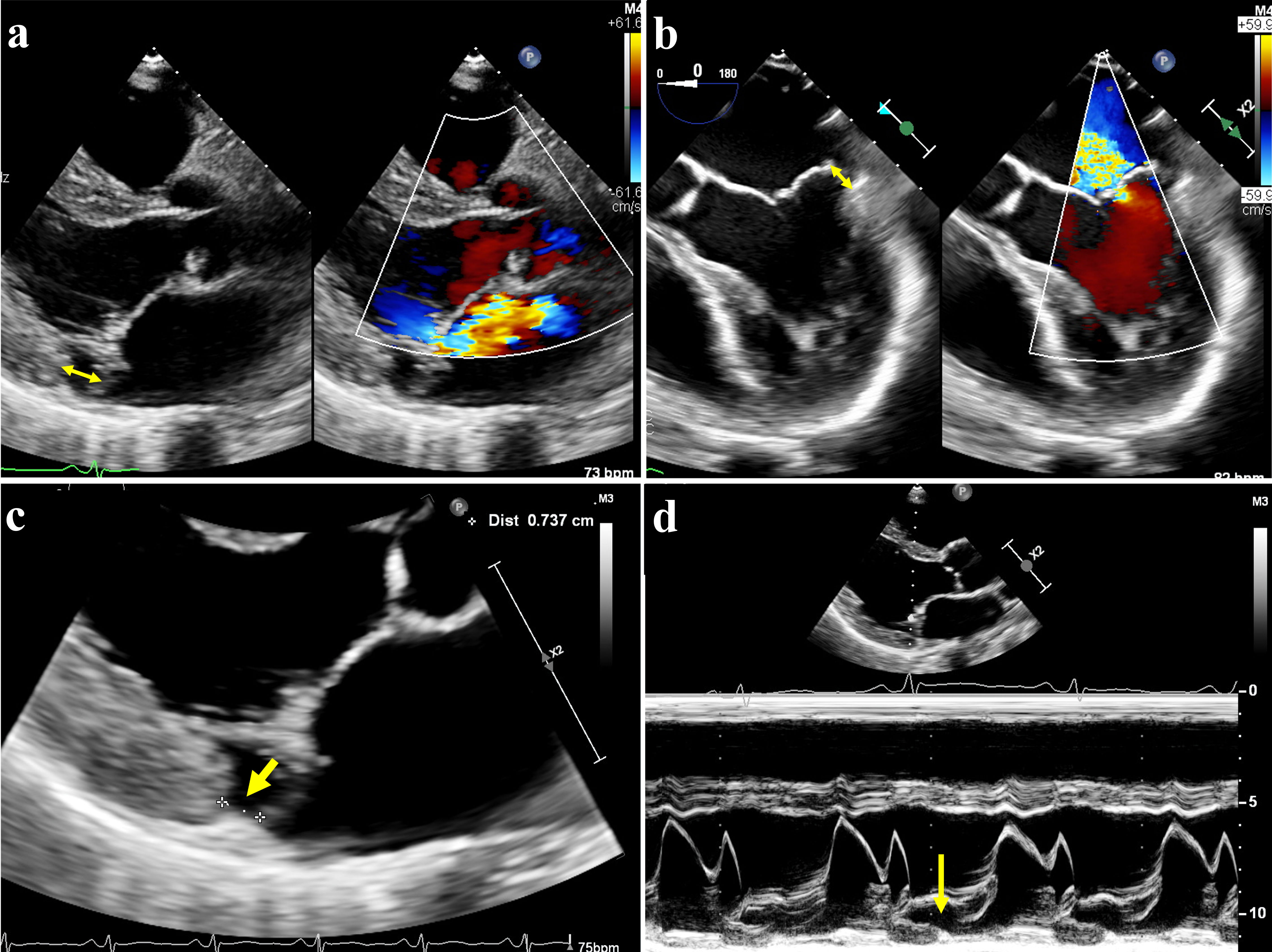 Click for large image | Figure 1. Mitral annular disjunction (MAD) on echocardiography. (a) Transthoracic echography (TTE) showed posterior leaflet prolapse with eccentric mitral regurgitation, with MAD seen (double-arrow). (b) Transesophageal echocardiography (TEE) of same patient also demonstrated MAD in systole (double-arrow). (c) MAD was demonstrated on parasternal long axis view on TTE, with the distance between mitral valve leaflet-atrial wall and left ventricle measured at 7 mm (arrow). (d) The hypermobile posterior annulus could be appreciated on the M-mode of TTE (arrow). |
In the TTE study of 979 patients of MVP with severe MR (including 637 cases of Barlow’s disease and 342 cases of fibroelastic deficiency) by Mantegazza et al [5], MAD was detected in 21.8% of patients with Barlow’s disease, versus 5.8% of patients with fibroelastic deficiency (P < 0.001). The maximum disjunction distance was 6.7 ± 2.2 mm, mainly located in P1 and P2 segments (69.2%) compared to P3 segment (37.7%) of the posterior leaflet.
In transoesophageal echocardiogram (TEE), the degree of annular disjunction could be measured in the four-chamber mid-esophageal view at 0° during systole. When applying a threshold of ≥ 5 mm by Eriksson et al [2], MAD was detected in 98% of patients with advanced Barlow’s disease, and the severity of the disjunction was significantly correlated with the number of prolapsing segments.
Real-time three-dimensional TEE provides more quantitative details for the spatial relation between the LV, LA and posterior leaflet attachment. In a total of 101 patients with MVP, the prevalence of MAD was 42% when using a threshold of > 5 mm, with mean disjunction distance of 8.9 mm and circumferential extension of 87±41° [6]. Paradoxical systolic expansion and flattening of the annulus were detected compared to patients without MAD, and the extent of the disjunction correlated significantly with larger regurgitant orifice.
Cardiac computed tomography (CCT) can also be utilized to identify MAD. In the retrospective study of preoperative CCT for 90 MVP cases by Putnam et al [7], MAD was prevalent in 20% of the patients. The disjunction was typically found adjacent to a prolapsed or flail segment in the posterior mitral leaflet. Seventy-five percent of the MAD cases had a maximum disjunction distance > 4.8 mm. The presence of MAD was found to be associated with female gender, smaller annulus size, and greater posterior leaflet length.
Cardiac magnetic resonance imaging (CMR) has been found to be able to detect significantly higher prevalence of MAD compared to TTE and TEE, with good agreement on MAD identification with TEE [8]. In the study of 116 patients with MAD by Dejgaard et al, the disjunction was identified exclusively along the posterior mitral leaflet, with a circumferential extension between 30° and 240° (with median of 150°) [9]. Additionally, CMR also provides valuable information regarding the presence and extent of myocardial fibrosis, which is common in patients with MVP (28-37%) [10, 11]. Myocardial fibrosis has been frequently detected near the mitral annulus in the basal LV wall, including the papillary muscles (PMs) and inferior wall. Late gadolinium enhancement (LGE) within the PMs and mitral valve apparatus has been found to be related pathophysiologically with arrhythmia [12]. Strain patterns can also be evaluated by CMR in the cases of MAD. In the case-control study of 63 patients by Wang et al [13], cases with MAD and MVP had lower basal longitudinal strain on TTE and lower magnitude in the basal inferolateral segments by circumferential and radial strain on CMR, compared to those with MVP but without MAD or controls. The abnormal strain patterns identified in the basal segments by CMR would be useful to detect regional LV dysfunction associated with MAD.
For comprehensive assessment of MAD, an integrated multi-imaging approach is recommended. Echocardiography including TTE and TEE remains to be a fundamental tool to assess the anatomic and hemodynamic features of the mitral valve disease. CCT and MRI would be helpful to identify the MAD with smaller length, to provide more anatomic details of the mitral apparatus, and to examine the extent of myocardial fibrosis and regional LV dysfunction.
| Clinical Implications of MAD | ▴Top |
MAD and MVP
MAD is now known to be frequently associated with MVP. In the systematic review including 19 studies of MAD by Bennett et al [14], the pooled prevalence of MAD was 32.6% among the patients with MVP and 50.8% among the subgroup of patients with Barlow’s disease. On the other hand, the prevalence of MVP was reported at 78% among patients with MAD [9].
Mantegazza et al found that significant MR occurred at an earlier age when MAD was present in patient with MVP [5]. MAD was seen more frequently in the cases of bileaflet prolapse (48.4% vs. 25.9%; P < 0.001) and Barlow’s disease (87.4% vs. 60.7%; P < 0.001), while with lower incidence in cases with chordal rupture (61.0% vs. 75.7%; P < 0.001).
In the analysis of 595 patients with isolated MVP [15], Essayagh et al identified MAD to be present in 31% of the cases, and 28% of the patients had severe MR. Advanced myxomatous valve disease with bileaflet involvement and significantly redundant leaflet tissue was independently associated with MAD. Symptoms of syncope or palpitation were more frequent in patients with MAD. Ventricular arrhythmia (VA) was correlated with more redundant or longer MAD, and larger ventricle size. Among the 183 patients (31%) who underwent mitral valve surgery (93% repair, 7% replacement), 63 patients had MAD. The echocardiography after surgery showed no MAD in 93% of the patients with MAD before operation. The association between arrhythmic event and MAD became weaker after mitral surgery, which was not affected by the extent of leaflet involvement or redundancy, or MR severity (P < 0.005).
Furthermore, Essayagh et al reported a study of 61 surgical MVP patients with MAD (8 ± 3 mm) present in 27 (44%) cases [16]. The mean effective regurgitant orifice area was similar compared to patients without MAD. MAD was more frequently seen in cases of bileaflet prolapse (52% vs. 18%, P = 0.004), and consistently involving P2 (P = 0.005). Patients with MAD were identified with larger annular areas, circumferences, and intercommissural diameters in diastole, compared to those without MAD (P ≤ 0.008). It is postulated that the MAD has resulted in considerable annular expansion during systole, thus affecting the leaflet coaptation, while the myocardial and annular slippage also stimulates vigorous ventricle function, leading to extensive myocardial fibrosis.
Although being frequently reported in cases with MVP, MAD had been also detected in patients with almost otherwise normal mitral valve. In the study of 718 consecutive cases referred for echocardiogram for any indication by Konda et al, 45 patients were identified with MAD [17]. The authors proposed three variations of MAD. Type I is with hypermobile mitral annulus but no distance of disjunction. Type II is with MAD distance < 5 mm, and type III is with MAD distance ≥ 5 mm. Among all the MAD cases, three (7%) patients did not have any MR, and 16 (36%) patients had only mild MR, with type I MAD. When comparing the tissue doppler imaging (TDI), the septal (12.0 ± 3.0 cm/s vs. 7.3 ± 2.9 cm/s, P < 0.0001), lateral (13.0 ± 3.4 cm/s vs. 9.2 ± 3.3 cm/s, P < 0.0001) and posterior (14.3 ± 3.2 cm/s vs. 9.5 ± 3.4 cm/s, P < 0.0001) velocities were significantly greater in patients with MAD than those without.
MAD and arrhythmic MVP
There is an increasing awareness of the occurrence of arrhythmia in patients with MVP (arrhythmic MVP). Patients could present with frequent (≥ 5%) premature ventricular contractions (PVCs), or complex VAs, without any other well-defined arrhythmic substrates except for MVP [18].
Two common phenotypes have been identified. The first group is patients with MVP and severe MR, with increased risk of mortality and sudden cardiac death (SCD), compared to general population. For this group of patients, MV surgery to correct the MR is beneficial to reduce the overall mortality [19] and risk of SCD [20]. The other group is severe myxomatous mitral valve disease with MVP, with variable MR severity. When there is no severe MR or LV dysfunction, the overall survival of patients with MVP was believed to be equivalent to that of the normal population. However, a small subset of patients has been reported with a malignant course, who would present with SCD without another relevant pathology other than MVP [21]. MAD is often detected in this clinical phenotype, with severe myxomatous degeneration, marked redundant leaflets, which is linked to higher incidence of VA.
The mechanism of VA in MVP has been proposed as an interplay of factors including myocardial fibrosis (substrate), ventricular ectopy (trigger), and transient modulators (hyperadrenergic state, hemodynamics, electrolytes) [22]. Detailed invasive voltage mapping in MVP patients with arrhythmias has correlated the origin of PVCs with the PMs, fascicular system, LV outflow tract and the mitral annulus. PVCs arising from Purkinje tissue could trigger ventricular fibrillation (VF) resulting in SCD [23]. Invasive voltage mapping of patients with bileaflet MVP also revealed fractionated, split and delayed Purkinje potentials, indicating abnormal tissue contributing in arrhythmogenesis and risk of SCD [24].
It has been found that MAD with a distance > 8.5 mm was associated with NSVT (odds ratio (OR): 10, 95% confidence interval (CI): 1.28 - 78.1) and the longitudinal extent of disjunction in the posterolateral wall (OR: 1.16; 95% CI: 1.02 - 1.33) was predictive of VAs. LGE in the anterolateral PM found in CMR was also strongly related to severe arrhythmias (OR: 7.35; 95% CI: 1.15 - 47.02) [14].
In the study of 89 patients with MVP by Essayagh et al, MAD was identified in 35% of the patients, more frequently seen in myxomatous disease, bileaflet prolapse, with higher incidence of NSVT, but not related to the severity of MR [25]. In their subsequent study of 595 consecutive MVP patients [26], frequent VAs were detected, including 9% of severe arrhythmia. Severe VAs was independently associated with the presence of MAD (P < 0.0001), but not with the severity of MR or LV function. Severe arrhythmia was also significantly associated with higher mortality, particularly under medical treatment (adjusted HR: 5.80; 95% CI: 2.75 - 12.23; P < 0.0001), but weaker after mitral operation (adjusted HR: 3.69; 95% CI: 0.93 - 14.74; P = 0.06).
The contribution of MAD to the occurrence of arrhythmia is likely due to the progressive fibrosis of mitral apparatus, which could be a result of mechanical stress exerted by the excessive mobile basal anterolateral and posterolateral LV segments. This can be demonstrated on echocardiogram as the Pickelhaube sign, with a spiked systolic high-velocity signal > 16 cm/s at the lateral mitral annulus on tissue Doppler, which resembles the spike that adorns the Pickelhaube helmet. It has been proposed that this sharp spike is resulted by the excessive tethering of the posterolateral LV wall due to the tugging of the posteromedial PM by the myxomatous prolapsing leaflets in mid-to-late systole. Pickelhaube sign has been proposed as a novel echocardiographic risk marker for malignant MVP syndrome [27]. Particularly, attention has been called for sonographers to interrogate the posterolateral annulus, where the highest Pickelhaube signal velocity has been identified in the majority of patients with bileaflet MVP [28].
In fact, MAD can be detected as early as in pediatric population, with the incidence as high as 50% in children with MVP. The presence of Pickelhaube sign and myxomatous mitral valve were found to be associated with significant VAs on 24-h Holter monitoring [29]. Although no difference in survival has been found in patients with MVP with or without MAD in the first 10 years, the occurrence of arrhythmia is, however, often delayed long after the diagnosis of MVP with MAD, and SCD or the excess mortality often happens even much later after the onset of severe arrhythmias [18]. Severe arrhythmia is definitely associated with significantly increased mortality in the long term. Arrhythmic MVP is a clinical phenotype dominated by MAD, marked leaflet redundancy, and electrophysiological abnormalities, with its strongly associated outcomes, which warrants early detection and careful risk stratification to guide its further management [26].
| Surgical Considerations in MAD | ▴Top |
MAD with severe MR
The presence of MAD should be examined routinely when assessing any case of MVP. The recognition of MAD is imperative in the surgical planning in MV surgery. With modification of repair techniques, complete resolution of MAD can be achieved in most patients after MV operation [2]. The annuloplasty ring or band should be sutured to affix the mitral annulus to the correct level of the myocardium in the LV, to obliterate the gap caused by the disjunction, rather than to be attached to LA wall (Fig. 2). The study of Essayagh et al has shown that the presence of MAD does not affect the repairability of prolapsed mitral valve per se [16]. MAD could recede after MV repair, and severe arrhythmia was observed with lower incidence post successful mitral surgery compared to those patients under medical treatment. Whether the persistence or recurrence of MAD after mitral surgery could result in further myocardial fibrosis and severe arrhythmia still remains unclear.
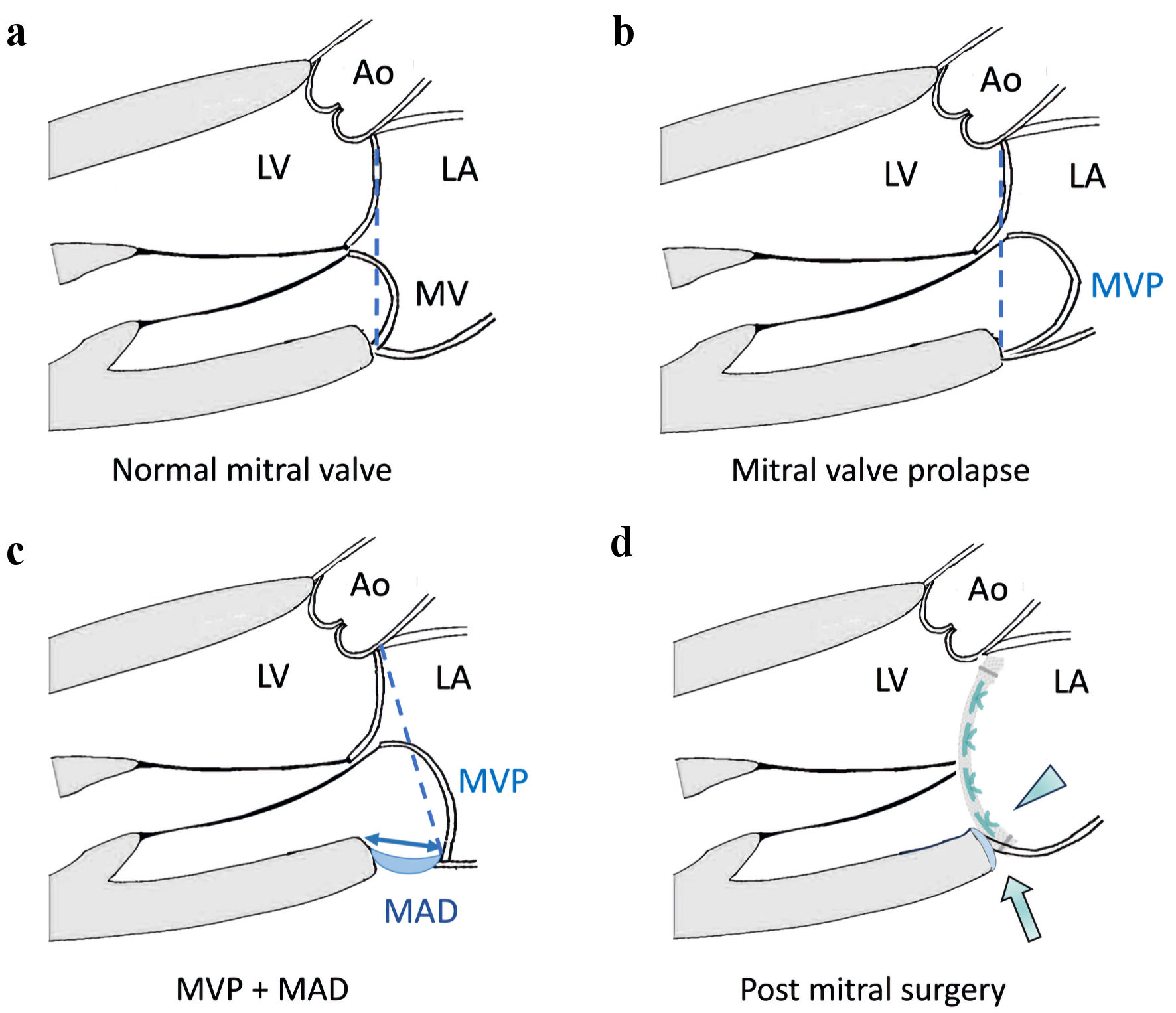 Click for large image | Figure 2. Illustrations of mitral annular disjunction (MAD) and surgical principles for mitral valve surgery. (a) Normal mitral valve: mitral annular plane is in its normal position (dashed line). (b) Mitral valve prolapse (MVP) without MAD: mitral annulus plane (dashed line) remains in its normal position despite prolapse of the mitral leaflet. (c) MVP with MAD: the mitral annulus is “dislocated” away from the normal left ventricle-left atrial (LV-LA) myocardium junction resulting in MAD (double-arrow), and the mitral annular plane (dashed line) has shifted towards LA. (d) MAD can be treated by mitral valve surgery, by suturing the prosthetic ring or valve (arrowhead) to the correct level of the LV-LA myocardial junction. The mitral annulus is stabilized, and the MAD can be obliterated after mitral surgery (arrow). Ao: aorta; LV: left ventricle; LA: left atrium; MV: mitral valve; MVP: mitral valve prolapse; MAD: mitral annular disjunction. |
Tirone David’s group used to propose to detach and reduce the posterior leaflet height before reattaching to the proximal musculature of the LV, followed by flexible annuloplasty ring. They also advocated the usage of artificial chordae to improve the long-term durability of the repair results [30]. Freedom from valve-related morbidity and mortality was achieved at 90±2% by 5 years and 78±4% by 10 years. They also found that the repair result in the case of Barlow’s disease and MAD seemed to be enduring, but not as good as the result in repairing an isolated prolapse of posterior leaflet. Myxomatous mitral valve disease was postulated to be genetically mediated disorder [31], and the pathologic expression is likely more pronounced in cases with incomplete annulus fibrosus, which is the case of MAD. This disjunction is further accentuated by the increased leaflet volume and resultant increase in tension. This may explain why 11.6% of the cases were detected with moderate MR on follow-up post mitral surgery, although only 3% of them needed reoperation for the recurrence of severe MR. It seems that MV repair in the case of MAD could slow down but could not stop the degenerative process completely.
Up to date, there is inadequate evidence to support which surgical approach (MV repair versus replacement) is more beneficial or effective to reduce the postoperative arrhythmic events per se. Both mitral annuloplasty (by using a prosthetic ring) and mitral valve replacement (MVR, by using a prosthetic valve) can correct the MAD by collapsing of the area of disjunction by either suturing the ring or the prosthesis to the LV myocardium. The resolution of MAD has been shown to reduce the arrhythmia burden in patients with MVP. Resolution of the Pickelhaube sign on echocardiogram has also been observed after a successful MV repair surgery [32]. Otherwise, the same surgical principles of MV surgery should be applied in the cases of severe MR with or without MAD. As it has been well established that for patients with degenerative MR, MV repair is associated with lower surgical mortality, durable long-term results with better survival, and fewer valve-related complications compared to MVR across all age groups, MV repair should remain as the first choice of surgical intervention when the mitral anatomy is favorable [33, 34]. When MV repair is not feasible, chord-preserving MVR would be the favored surgical approach.
MAD with arrhythmia
PVCs originating from PMs are common in patients with MVP, which can trigger malignant VAs. Catheter ablation by radiofrequency or cryoenergy has been established as an effective percutaneous intervention to treat the PM-PVCs in advanced MVP, however, with only modest (60-80%) success rates due to the technical challenge in mapping and ablating of PMs due to its complex anatomy, which often requires repeated procedures [23].
Successful correction of severe MR in MV surgery has been proved to reduce the arrhythmia burden in patients with degenerative MR due to MVP [35, 36]. Surgical ablation has been used extensively to treat atrial fibrillation at the time of cardiac surgery, with established long-term results. However, surgical ablation for VA has so far been only limited to treating the scar-related re-entrant VAs and outflow tract arrhythmias [37].
Recently, cases of surgical cryoablation of the PMs under direct vision during MV surgery have been reported [38, 39]. The origin of the PVCs at the PM-region was assessed based on 12-lead electrocardiogram. Cryoablation was then performed at both the base and tip of the target PM, followed by MV surgery. PVC burden was reduced > 90% in short-term follow-up, although the long-term safety and efficacy still need to be examined in a larger group of cases. Randomized trial is needed to assess if the additional PM ablation really contributes to further reduction in the PVC burden postoperatively compared to a successful mitral surgery to treat the severe MR alone.
The surgical indications in MVP patients with moderate MR are yet to be determined. However, in MVP cases with malignant arrhythmia but less than severe MR, mitral surgery may have a role in suppressing the progress of valve prolapse to reduce the arrhythmia burden, thus, to prevent SCD. This group of patients require close follow-up by a multidisciplinary team involving mitral valve surgeon and cardiac electrophysiologist. The knowledge gaps will need to be filled in by further clinical trials to define the role of surgery in the treatment of advanced MVP with different grades of MR.
| Conclusions | ▴Top |
The phenotypes of MAD, MVP, myxomatous mitral valve degeneration are likely intertwining but comprising the components of the same clinical spectrum, which over time contributes strongly and independently to the occurrence of VAs, with associated risk of SCD. As the current link between MVP with MAD and potential future VAs are better established, the awareness and early detection of these clinical entities are becoming more and more important, to guide the strategies for risk stratification and further intervention. MV surgery (to repair if possible) remains the standard of care for patients with severe degenerative MR. The surgical correction of MAD seemed to be beneficial to reduce VA burden after surgery. However, the role of MV surgery in treating arrhythmogenic MVP with less severe MR and the role of surgical cryoablation of the PMs have yet to be established, with the questions of the benefit in reducing risk of SCD. The surgical strategies with their long-term outcomes need to be further assessed in larger prospective studies.
Acknowledgments
None to declare.
Financial Disclosure
The authors declare that they do not have a financial relationship with any commercial entity that has an interest in the subject of this manuscript.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Author Contributions
Conception and design: L. Zhu and YL Chua. Data collection and analysis: L Zhu. Administrative support: L Zhu. Manuscript writing and final approval of manuscript: L Zhu and YL Chua.
Data Availability
The authors declare that data supporting this study are available within the article.
Abbreviations
MAD: mitral annular disjunction; MV: mitral valve; MVP: mitral valve prolapse; NSVT: non-sustained ventricular tachycardia; MR: mitral regurgitation; TTE: transthoracic echocardiogram; TEE: transoesophageal echocardiogram; CCT: cardiac computed tomography; CMR: cardiac magnetic resonance imaging; LGE: late gadolinium enhancement; TDI: tissue Doppler imaging; PVC: premature ventricular contractions; VA: ventricular arrhythmia; VT: ventricular tachycardia; VF: ventricular fibrillation; SCD: sudden cardiac death; MVR: mitral valve replacement; PM: papillary muscle
| References | ▴Top |
- Hutchins GM, Moore GW, Skoog DK. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N Engl J Med. 1986;314(9):535-540.
doi pubmed - Eriksson MJ, Bitkover CY, Omran AS, David TE, Ivanov J, Ali MJ, Woo A, et al. Mitral annular disjunction in advanced myxomatous mitral valve disease: echocardiographic detection and surgical correction. J Am Soc Echocardiogr. 2005;18(10):1014-1022.
doi pubmed - Carmo P, Andrade MJ, Aguiar C, Rodrigues R, Gouveia R, Silva JA. Mitral annular disjunction in myxomatous mitral valve disease: a relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc Ultrasound. 2010;8:53.
doi pubmed pmc - Wunderlich NC, Ho SY, Flint N, Siegel RJ. Myxomatous mitral valve disease with mitral valve prolapse and mitral annular disjunction: clinical and functional significance of the coincidence. J Cardiovasc Dev Dis. 2021;8(2):1-15.
doi pubmed pmc - Mantegazza V, Tamborini G, Muratori M, Gripari P, Fusini L, Italiano G, Volpato V, et al. Mitral annular disjunction in a large cohort of patients with mitral valve prolapse and significant regurgitation. JACC Cardiovasc Imaging. 2019;12(11 Pt 1):2278-2280.
doi pubmed - Lee AP, Jin CN, Fan Y, Wong RHL, Underwood MJ, Wan S. Functional Implication of Mitral annular disjunction in mitral valve prolapse: a quantitative dynamic 3D echocardiographic study. JACC Cardiovasc Imaging. 2017;10(12):1424-1433.
doi pubmed - Putnam AJ, Kebed K, Mor-Avi V, Rashedi N, Sun D, Patel B, Balkhy H, et al. Prevalence of mitral annular disjunction in patients with mitral valve prolapse and severe regurgitation. Int J Cardiovasc Imaging. 2020;36(7):1363-1370.
doi pubmed pmc - Mantegazza V, Volpato V, Gripari P, Ghulam Ali S, Fusini L, Italiano G, Muratori M, et al. Multimodality imaging assessment of mitral annular disjunction in mitral valve prolapse. Heart. 2021;107(1):25-32.
doi pubmed - Dejgaard LA, Skjolsvik ET, Lie OH, Ribe M, Stokke MK, Hegbom F, Scheirlynck ES, et al. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol. 2018;72(14):1600-1609.
doi pubmed - Kitkungvan D, Nabi F, Kim RJ, Bonow RO, Khan MA, Xu J, Little SH, et al. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol. 2018;72(8):823-834.
doi pubmed - Constant Dit Beaufils AL, Huttin O, Jobbe-Duval A, Senage T, Filippetti L, Piriou N, Cueff C, et al. Replacement myocardial fibrosis in patients with mitral valve prolapse: relation to mitral regurgitation, ventricular remodeling, and arrhythmia. Circulation. 2021;143(18):1763-1774.
doi pubmed - Perazzolo Marra M, Basso C. Mechanical dispersion and arrhythmic mitral valve prolapse: substrate and trigger in electrical instability. Heart. 2019;105(14):1053-1054.
doi pubmed - Wang TKM, Kwon DH, Abou-Hassan O, Chetrit M, Harb SC, Patel D, Kalahasti V, et al. Strain evaluation for mitral annular disjunction by echocardiography and magnetic resonance imaging: A case-control study. Int J Cardiol. 2021;334:154-156.
doi pubmed - Bennett S, Thamman R, Griffiths T, Oxley C, Khan JN, Phan T, Patwala A, et al. Mitral annular disjunction: A systematic review of the literature. Echocardiography. 2019;36(8):1549-1558.
doi pubmed - Essayagh B, Sabbag A, Antoine C, Benfari G, Batista R, Yang LT, Maalouf J, et al. The mitral annular disjunction of mitral valve prolapse: presentation and outcome. JACC Cardiovasc Imaging. 2021;14(11):2073-2087.
doi pubmed - Essayagh B, Mantovani F, Benfari G, Maalouf JF, Mankad S, Thapa P, Michelena HI, et al. Mitral annular disjunction of degenerative mitral regurgitation: three-dimensional evaluation and implications for mitral repair. J Am Soc Echocardiogr. 2022;35(2):165-175.
doi pubmed - Konda T, Tani T, Furukawa Y. Mitral annular disjunction in consecutive cases: Echocardiographic detection. J Am Coll Cardiol. 2013;61(10):E1046.
- Sabbag A, Essayagh B, Barrera JDR, Basso C, Berni A, Cosyns B, Deharo JC, et al. EHRA expert consensus statement on arrhythmic mitral valve prolapse and mitral annular disjunction complex in collaboration with the ESC Council on valvular heart disease and the European Association of Cardiovascular Imaging endorsed cby the Heart Rhythm Society, by the Asia Pacific Heart Rhythm Society, and by the Latin American Heart Rhythm Society. Europace. 2022;24(12):1981-2003.
doi pubmed - Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, Scott C, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352(9):875-883.
doi pubmed - Grigioni F, Enriquez-Sarano M, Ling LH, Bailey KR, Seward JB, Tajik AJ, Frye RL. Sudden death in mitral regurgitation due to flail leaflet. J Am Coll Cardiol. 1999;34(7):2078-2085.
doi pubmed - Basso C, Perazzolo Marra M, Rizzo S, De Lazzari M, Giorgi B, Cipriani A, Frigo AC, et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 2015;132(7):556-566.
doi pubmed - Miller MA, Dukkipati SR, Turagam M, Liao SL, Adams DH, Reddy VY. Arrhythmic mitral valve prolapse: JACC review topic of the week. J Am Coll Cardiol. 2018;72(23 Pt A):2904-2914.
doi pubmed - Enriquez A, Shirai Y, Huang J, Liang J, Briceno D, Hayashi T, Muser D, et al. Papillary muscle ventricular arrhythmias in patients with arrhythmic mitral valve prolapse: Electrophysiologic substrate and catheter ablation outcomes. J Cardiovasc Electrophysiol. 2019;30(6):827-835.
doi pubmed - Syed FF, Ackerman MJ, McLeod CJ, Kapa S, Mulpuru SK, Sriram CS, Cannon BC, et al. Sites of successful ventricular fibrillation ablation in bileaflet mitral valve prolapse syndrome. Circ Arrhythm Electrophysiol. 2016;9(5):e004005.
doi pubmed - Essayagh B, Iacuzio L, Civaia F, Avierinos JF, Tribouilloy C, Levy F. Usefulness of 3-tesla cardiac magnetic resonance to detect mitral annular disjunction in patients with mitral valve prolapse. Am J Cardiol. 2019;124(11):1725-1730.
doi pubmed - Essayagh B, Sabbag A, Antoine C, Benfari G, Yang LT, Maalouf J, Asirvatham S, et al. Presentation and outcome of arrhythmic mitral valve prolapse. J Am Coll Cardiol. 2020;76(6):637-649.
doi pubmed - Muthukumar L, Rahman F, Jan MF, Shaikh A, Kalvin L, Dhala A, Jahangir A, et al. The Pickelhaube sign: novel echocardiographic risk marker for malignant mitral valve prolapse syndrome. JACC Cardiovasc Imaging. 2017;10(9):1078-1080.
doi pubmed - Ignatowski D, Schweitzer M, Pesek K, Jain R, Muthukumar L, Khandheria BK, Tajik AJ. Pickelhaube spike, a high-risk marker for bileaflet myxomatous mitral valve prolapse: sonographer's quest for the highest spike. J Am Soc Echocardiogr. 2020;33(5):639-640.
doi pubmed - Vaksmann G, Bouzguenda I, Guillaume MP, Gras P, Silvestri V, Richard A. Mitral annular disjunction and Pickelhaube sign in children with mitral valve prolapse: A prospective cohort study. Arch Cardiovasc Dis. 2023;116(11):514-522.
doi pubmed - Newcomb AE, David TE, Lad VS, Bobiarski J, Armstrong S, Maganti M. Mitral valve repair for advanced myxomatous degeneration with posterior displacement of the mitral annulus. J Thorac Cardiovasc Surg. 2008;136(6):1503-1509.
doi pubmed - Kyndt F, Gueffet JP, Probst V, Jaafar P, Legendre A, Le Bouffant F, Toquet C, et al. Mutations in the gene encoding filamin A as a cause for familial cardiac valvular dystrophy. Circulation. 2007;115(1):40-49.
doi pubmed - Weiner MM, Boateng P, Pandis D, Miller MA, Adams DH. Impact of mitral valve repair on the Pickelhaube sign. Eur Heart J. 2019;40(27):2267.
doi pubmed - Lazam S, Vanoverschelde JL, Tribouilloy C, Grigioni F, Suri RM, Avierinos JF, de Meester C, et al. Twenty-year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation: analysis of a large, prospective, multicenter, international registry. Circulation. 2017;135(5):410-422.
doi pubmed - Jung JC, Jang MJ, Hwang HY. Meta-analysis comparing mitral valve repair versus replacement for degenerative mitral regurgitation across all ages. Am J Cardiol. 2019;123(3):446-453.
doi pubmed - Alqarawi W, Birnie DH, Burwash IG. Mitral valve repair results in suppression of ventricular arrhythmias and normalization of repolarization abnormalities in mitral valve prolapse. HeartRhythm Case Rep. 2018;4(5):191-194.
doi pubmed pmc - Vaidya VR, DeSimone CV, Damle N, Naksuk N, Syed FF, Ackerman MJ, Ponamgi SP, et al. Reduction in malignant ventricular arrhythmia and appropriate shocks following surgical correction of bileaflet mitral valve prolapse. J Interv Card Electrophysiol. 2016;46(2):137-143.
doi pubmed pmc - Choi EK, Nagashima K, Lin KY, Kumar S, Barbhaiya CR, Baldinger SH, Reichlin T, et al. Surgical cryoablation for ventricular tachyarrhythmia arising from the left ventricular outflow tract region. Heart Rhythm. 2015;12(6):1128-1136.
doi pubmed - El-Eshmawi A, Pandis D, Miller MA, Boateng P, Dukkipati SR, Reddy VY, Adams DH. Surgical cryoablation of papillary muscle PVCs during mitral valve surgery: therapeutic consideration for malignant MVP. J Am Coll Cardiol. 2020;76(25):3061-3062.
doi pubmed - Vohra J, Morton JB, Morgan J, Tatoulis J. Cryoablation of Papillary Muscles at Surgery for Malignant Ventricular Arrhythmias Due to Mitral Valve Prolapse. Heart Lung Circ. 2022;31(9):1285-1290.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


