| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 15, Number 1, February 2024, pages 37-46
Characteristics and Outcomes of Atrial Fibrillation in Chronic Heart Failure Patients: A Comprehensive Analysis of the Colombian Heart Failure Registry
Alex Rivera-Toquicaa, b, c, Clara Saldarriagad, Jannes Buelvas-Herazoe, Balkis Rolongf, Fernando Manzur-Jating, Jose Ignacio Mosquera-Jimenezh, Oscar Alfredo Pacheco-Jimenezi, Alvaro Hernan Rodriguez-Ceronj, Patricia Rodriguez-Gomezk, Fernando Rivera-Toquical, m, Guillermo Trout-Guardiola Gn, Marco Antonio De Leon-Espitiao, Edgar Eduardo Castro-Osoriop, Luis Eduardo Echeverriaq, Juan Esteban Gomez-Mesar, s, t
aDepartment of Cardiology, Centro Medico para el Corazon, Pereira, Colombia
bDepartment of Cardiology, Clinica Los Rosales S.A., Pereira, Colombia
cDepartment of Cardiology, Universidad Tecnologica de Pereira, Pereira, Colombia
dDepartment of Cardiology, Clinica Cardio VID, Medellin, Colombia
eDepartment of Cardiology, Cardiodiagnostico, Barranquilla, Colombia
fDepartment of Cardiology, Cardiologia Integral, Barranquilla, Colombia
gDepartment of Cardiology, Universidad de Cartagena, Cartagena, Colombia
hDepartment of Cardiology, Clinica Santa Gracia (Dumian Medical S.A.S), Popayan, Colombia
iDepartment of Cardiology, Hospital Universitario Mayor - Mederi, Bogota, Colombia
jDepartment of Cardiology, Hospital Cardiovascular de Cundinamarca S.A., Soacha, Colombia
kDepartment of Cardiology, Unidad Cardiologica de Cartagena, Cartagena, Colombia
lDepartment of Internal Medicine, Clinica Los Rosales S.A., Pereira, Colombia
mDepartment of Internal Medicine, IPS Virrey Solis, Pereira, Colombia
nDepartment of Cardiology, GroupSalud IPS, Santa Marta, Colombia
oDepartment of Cardiology, Cardio Care Colombia S.A.S., Monteria, Colombia
pDepartment of Cardiology, S.E.S. Hospital Universitario de Caldas, Manizales, Colombia
qDepartment of Cardiology, Fundacion Cardiovascular de Colombia, Floridablanca, Colombia
rDepartment of Cardiology, Fundacion Valle del Lili, Cali, Colombia
sDepartment of Health Sciences, Universidad Icesi, Cali, Colombia
tCorresponding Author: Juan Esteban Gomez-Mesa, Department of Cardiology, Fundacion Valle del Lili, Cali, Colombia
Manuscript submitted October 30, 2023, accepted December 2, 2023, published online February 28, 2024
Short title: Atrial Fibrillation in Chronic Heart Failure
doi: https://doi.org/10.14740/cr1589
| Abstract | ▴Top |
Background: Heart failure (HF) and atrial fibrillation (AF) represent conditions that commonly coexist. The impact of AF in HF has yet to be well studied in Latin America. This study aimed to characterize the sociodemographic and clinical features, along with patients’ outcomes with AF and HF from the Colombian Heart Failure Registry (RECOLFACA).
Methods: Patients with ambulatory HF and AF were included in RECOLFACA, mainly with persistent or permanent AF. A 6-month follow-up was performed. Primary outcome was all-cause mortality. To assess the impact of AF on mortality, we used a logistic regression model. A P value of < 0.05 was considered significant. All statistical tests were two-tailed.
Results: Of 2,528 patients with HF in the registry, 2,514 records included information regarding AF diagnosis. Five hundred sixty (22.3%) were in AF (mean age 73 ± 11, 56% men), while 1,954 had no AF (mean age 66 ± 14 years, 58% men). Patients with AF were significantly older and had a different profile of comorbidities and implanted devices compared to non-AF patients. Moreover, AF diagnosis was associated with lower quality of life score (EuroQol-5D), mainly in mobility, personal care, and daily activity. AF was prevalent in patients with preserved ejection fraction (EF), while no significant differences in N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels were observed. Although higher mortality was observed in the AF group compared to individuals without AF (8.9% vs. 6.1%, respectively; P = 0.016), this association lost statistical significance after adjusting by age in a multivariate regression model (odds ratio (OR): 1.35; 95% confidence interval (CI): 0.95 - 1.92).
Conclusions: AF is more prevalent in HF patients with higher EF, lower quality of life and different clinical profiles. Similar HF severity and non-independent association with mortality were observed in our cohort. These results emphasize the need for an improved understanding of the AF and HF coexistence phenomenon.
Keywords: Heart Failure; Atrial fibrillation; Ejection fraction; Mortality; Registry; Comorbidity; Colombia; Treatment
| Introduction | ▴Top |
Heart failure (HF) and atrial fibrillation (AF) represent prevalent conditions that commonly coexist, mainly derived from the mutual pathophysiological pathways on both diseases [1]. On one side, HF represents one of the most chronic non-transmissible diseases, with an estimated prevalence of around 100 cases per 1,000 population in individuals over 65 years, with about 5.7 million Americans over 20 years of HF diagnosis [2]. On the other side, AF is the most frequently observed type of arrhythmia in general clinical practice [3]. AF prevalence in the United States has been estimated at around 2.6 to even 6.1 million, suggesting an increase of 2.5 times by 2050 [2, 4]. Added to their high prevalence, both AF and HF carry a significant burden of healthcare costs and morbimortality worldwide [5-7].
The close interrelationship between AF and HF was initially suggested by the results of the Framingham study, which reported a higher incidence of HF in AF patients (33 per 1,000 person-years) compared to those without AF and a high incidence of AF in patients with HF [8]. Furthermore, the coexistence of these two conditions was associated with increased mortality, especially in AF patients who subsequently developed HF [8]. After this, several studies have aimed to understand the prognostic significance of AF in patients with HF; nevertheless, there is still controversy regarding the role of AF as a risk factor for adverse outcomes in the context of HF [9]. The present study aimed to characterize the sociodemographic and clinical features, and outcomes of patients with AF and HF from the Colombian Heart Failure Registry (RECOLFACA).
| Materials and Methods | ▴Top |
Study design and population
RECOLFACA is a prospective cohort study that enrolled patients with a clinical diagnosis of HF based on international guidelines, from 60 medical institutions in Colombia. Recruitment period comprehended between February 2017 and October 2019. A 6-month follow-up after recruitment was performed. Details on inclusion and exclusion criteria are described elsewhere [10, 11]. Our study was approved by the Ethics Committee of the Fundacion Valle del Lili under act number 174-2017. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Data collection and outcomes
We registered all sociodemographic, clinical, and laboratory data at baseline. The diagnosis of AF was based on a 12-lead electrocardiogram (ECG) or previous documentation of this condition in the clinical record, which could have led to the possibility of missing patients with paroxysmal AF. HF severity was evaluated using the American Heart Association (AHA)/American College of Cardiology (ACC) stages stratification and the New York Heart Association (NYHA) classification. Description of all comorbidities assessed can be found in a previous report [10]. We considered triple therapy for HF treatment as the presence of the prescription of an angiotensin receptor blocker (ARB) or angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor neprilysin inhibitor (ARNI), plus a mineralocorticoid receptor antagonist (MRA) and a beta-blocker. In this study we present the data from the first follow-up performed 6 months post-enrollment into RECOLFACA (median follow-up time was 215 days).
Statistical analysis
At first, the total sample was divided into two groups (AF vs. non-AF patients). Continuous variables were reported as medians and quartiles, while categorical variables as proportions and absolute counts. Pearson’s Chi-squared, Fisher’s exact test or Mann-Whitney U test were used to find differences between groups according to the type of variable analyzed. The cumulative incidence of the mortality events was assessed with 95% confidence intervals (CIs). A multivariable logistic regression model was fitted to evaluate the prognostic role of AF diagnosis. A P value of < 0.05 (two-tailed test) was considered statistically significant. Statistical Package STATA version 15 (Station College, Texas, USA) was used for all statistical analyses.
| Results | ▴Top |
Of 2,528 patients in the RECOLFACA between 2017 and 2019, 2,514 records included information regarding AF diagnosis. The prevalence of AF among these patients was 22.3% (n = 560). Moreover, from a total of 66 patients (2.6% of the total) with a diagnosis of tachycardia-induced cardiomyopathy as HF etiology, 53 (80.3%) had AF.
Sociodemographic factors and comorbidities
No significant differences regarding sex and population were observed. Patients with AF were significantly older and had substantially higher rates of arterial hypertension, chronic obstructive pulmonary disease, thyroid disease, chronic kidney disease, and valvular disease. On the other hand, patients without AF were most frequently diagnosed with type 2 diabetes mellitus (Table 1).
 Click to view | Table 1. Sociodemographic and Clinical Characteristics Based on Atrial Fibrillation Diagnosis |
Clinical, laboratory, and echocardiographic differences
There were no significant differences regarding NYHA and AHA/ACC classifications between both groups, and they also had similar heart rates and QRS duration. On the other hand, AF diagnosis was associated with a significantly lower quality of life (QoL) score (Euro Qol-5D), mainly in the areas of mobility, personal care, and daily activity. This difference in the QoL score was still present even after accounting for differences in age, sex, and chronic kidney disease (variables also associated with QoL). Although the rates of an implantable cardioverter defibrillator (ICD) were similar between the two groups, patients with AF had a higher rate of ICD with cardiac resynchronization therapy (CRT) and a higher rate of implanted pacemakers (Table 1).
Regarding echocardiographic measures, the prevalence of HF with reduced ejection fraction (HFrEF) (< 40%) was significantly lower in AF patients (50.9% vs. 55.8% in non-AF patients, P = 0.040). AF patients reported a considerably lower diastolic diameter of the left ventricle while reporting a higher rate of pulmonary hypertension. Finally, AF patients had substantially lower creatinine values; the glomerular filtration rate was also lower in this group, while the blood urea nitrogen value was considerably higher. No significant differences in N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels were observed (Table 1).
HF treatment
Patients with AF had similar prescription rates of ARNI/ACEI/ARB and MRAs compared to non-AF patients. However, they were most frequently prescribed with beta-blockers, diuretics, and digoxin (Fig. 1). Finally, 93% of the total AF patients had at least one rate-control drug prescribed. Unfortunately, we could not assess the prescription of rhythm control medications, as the RECOLFACA registry did not include information regarding this type of drug.
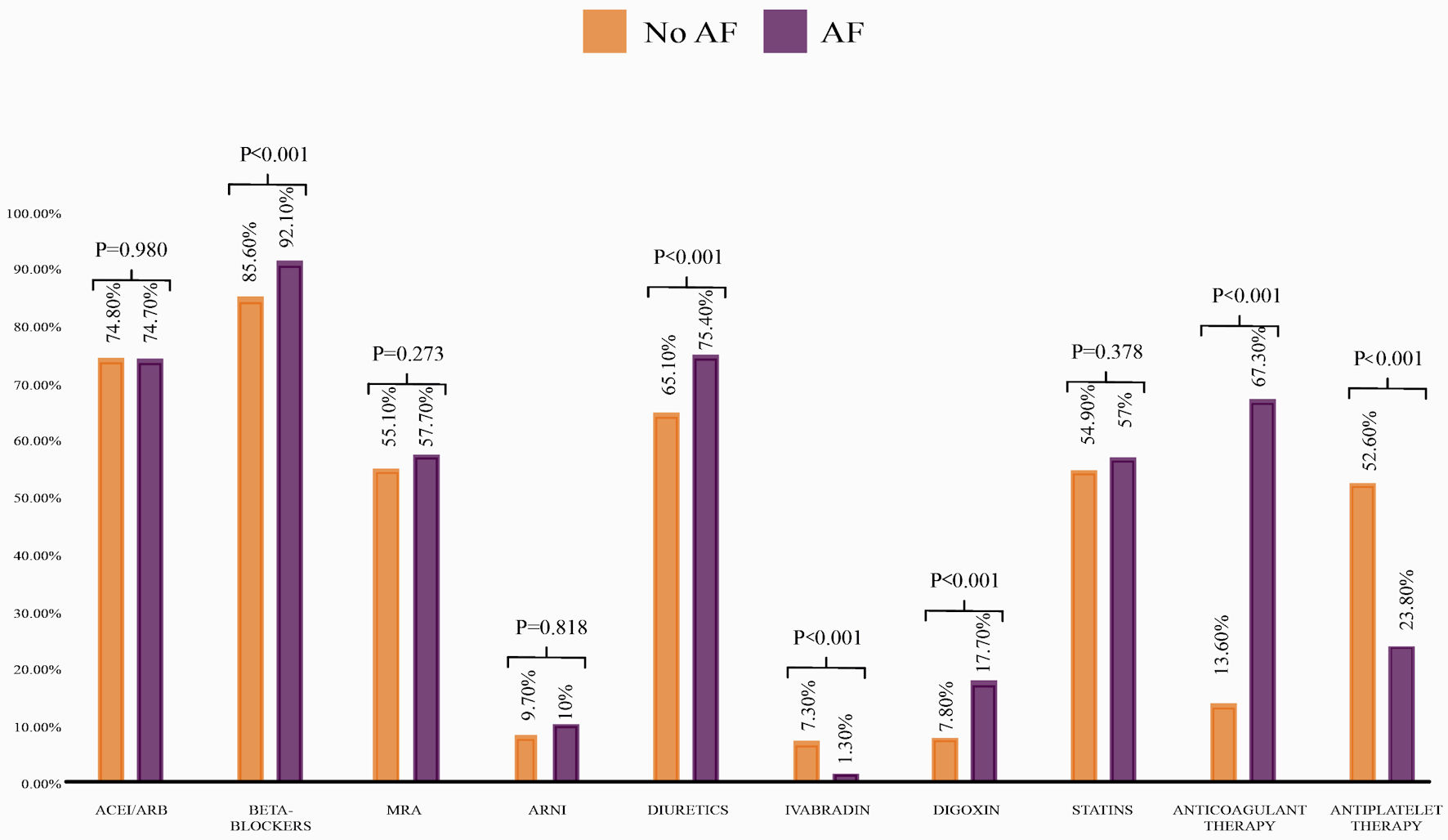 Click for large image | Figure 1. Prescription rates of HF medications based on AF diagnosis. ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; ARNI: angiotensin receptor neprilysin inhibitor; AF: atrial fibrillation; HF: heart failure; MRA: mineralocorticoid receptor antagonist. |
Mortality
During the follow-up we registered the death of 170 patients (6.76%), representing a mortality rate of 0.29 per 1,000 person-years (95% CI: 25.4 - 34.5). The AF group had significantly higher mortality than individuals without AF (8.9% vs. 6.1%, respectively, P = 0.016). However, this association was insignificant after age adjustment in a multivariate logistic regression model (odds ratio (OR): 1.35; 95% CI: 0.95 - 1.92).
AF and left ventricle ejection fraction (LVEF)
Prevalence of AF varies according to the LVEF, with a higher trend in its prevalence with higher values of LVEF. In HFrEF (< 40% LVEF), it is 20.7%; in HF with mildly reduced ejection fraction (HFmrEF) (41-49% LVEF), it is 22.6% and in HF with preserved ejection fraction (HFpEF) (> 50% LVEF), it is 24.8%.
Patients with AF and HFrEF or HFmrEF were significantly younger, had lower rates of thyroid disease, and were most frequently diagnosed with Chagas disease than patients with AF and HFpEF (Table 2). There were no significant differences in HF etiology between both groups except for chagasic (P = 0.014) and valvular (P = 0.027) etiology. There were no significant differences in the prescription rates of ACEI or ARB between the two groups. Interestingly, the beta-blocker prescription was not significantly higher in the HFrEF group. Finally, only MRAs prescription was higher in patients with HFrEF (Table 2). Mortality was similar in patients with AF despite the ejection fraction classification (HFpEF: 8.4% vs. HFrEF: 9.5%, P = 0.645). Nevertheless, AF diagnosis was associated with an increased mortality risk in HFrEF patients (OR: 1.71; 95% CI: 1.07 - 2.73), while no significant difference was observed in individuals with HFpEF (OR: 1.29; 95% CI: 0.78 - 2.14).
 Click to view | Table 2. Sociodemographic and Clinical Characteristics of Patients With HF and Atrial Fibrillation Based on Ejection Fraction |
| Discussion | ▴Top |
In the present study, we observed a prevalence of AF in patients with HF of 22.3%, highlighting essential sociodemographic, clinical, medication prescriptions, and laboratory differences between patients with and without AF. Moreover, patients with AF had a significantly higher mortality rate than non-AF individuals; however, this difference lost statistical significance when adjusted by age (as patients in the AF group were significantly older).
The complex inter-relationship between HF and AF has been a matter of interest during the last decades. Initial study results revealed the increased risk of complications attributed to the simultaneous presence of these two entities [8]. Since 1937, causal associations have been proposed when addressing the AF-HF interplay [12]. At first, AF can induce the appearance of HF due to an increase in the ventricular rate, a loss of atrial systole, increased irregularity of the ventricular response, poorly controlled ventricular rates, and worsened regurgitation of the mitral and tricuspid valves, finally leading to a reduction of the cardiac output [13, 14]. All of these factors are responsible for the development of HF, better known as tachycardia-induced cardiomyopathy, from which AF represents the most common etiology [9].
Conversely, HF promotes atrial changes that predispose the development of AF, mainly through processes such as the dysregulation of intracellular calcium, neuroendocrine dysfunction, and the elevation of cardiac filling pressures, among others [14]. The resulting increase in atrial stretch due to increased volumes and pressures promotes the activation of ionic currents, which favor alterations in physiological conduction pathways [15]. Finally, increased interstitial fibrosis of the atria has been consistently observed during HF in animal models, thus creating a relevant substrate for AF [16].
The prevalence of AF observed in the present study is similar to that reported in other registry-based studies and randomized clinical trials [13, 17-25]. However, substantial heterogeneity in the prevalence data was observed in the literature, ranging from 6% in the Studies of Left Ventricular Dysfunction (SOLVD) trials to 35% in the Japanese Cardiac Registry of HF [13, 24]. Furthermore, the higher prevalence of AF in patients with HFpEF compared to those with HFrEF observed in our study has also been described in other clinical studies [26-30], potentially being attributed to common risk factors for HFpEF and AF; however, this difference in our study was small and probably not clinically significant. The reasons behind the differential profile of AF by LVEF classification are still unclear [26].
Furthermore, considering the pathophysiological background, the coexistence of these two entities could also increase the severity of the cardiac involvement, thus, increasing the risk of adverse outcomes [8]. Several randomized clinical trials have evaluated the prognostic value of AF in the context of HF. For example, the SOLVD trial observed that AF was an independent predictor for all-cause mortality, being this effect mainly due to an increase in the risk of pump failure [13]. Similarly, in the Valsartan in Acute Myocardial Infarction (VALIANT), AF was associated with a higher risk of long-term morbidity and mortality in patients with myocardial infarction complicated by HF [22]. This added risk has also been observed in patients with HFpEF, as evidenced in the study of Aronow et al [31], in which patients with prior myocardial infarction and HF diagnosed with AF had a significantly higher 6-month mortality rate than those in sinus rhythm [31]. In the present study, patients with AF and HFrEF had a higher mortality risk than those with HFrEF without AF; nonetheless, AF was not an independent predictor of mortality, as it lost statistical significance as a risk factor after adjusting by age. Several studies have also suggested that AF may not confer a higher risk of mortality in the context of AF, while others report an increased risk of this adverse outcome only in HFpEF patients. Nevertheless, an adjusted meta-analysis of 16 studies (nine observational studies and seven randomized trials) assessing 53,969 patients suggested that patients with AF had a worse prognosis irrespective of the systolic function, with an increased risk of mortality both in the randomized trials (OR: 1.40, 95% CI: 1.32 - 1.48) and observational studies (OR: 1.14, 95% CI: 1.03 - 1.26). The reason behind the lack of significance in our study may be the small sample size, along with the short follow-up. We expect to reevaluate the prognostic impact of AF as the RECOLFACA registry continues the follow-up of the enrolled patients.
Study limitations
First, the RECOLFACA registry did not include information regarding rhythm control therapy, or the type of anticoagulant treatment prescribed. Second, the present study should have accounted for several potential confounders. Nonetheless, we intended to overcome this limitation by including prior medical history data, sociodemographic variables, echocardiographic measures, laboratory tests, and device therapy information. Third, the way of diagnosing AF used in this study might have induced a potential of missing patients with paroxysmal AF. Fourth, this study was conducted between 2017 and 2019, which explains the low percentage of patients receiving ARNI and the absence of information on sodium-glucose cotransporter 2 inhibitor (SGLT2i) prescriptions in patients with HF. When the registry ended recruitment (2019), SGLT2i were not approved for this indication in our country. These two conditions could affect the clinical outcomes of patients.
Conclusions
In this study, AF represents a common comorbidity in patients with HF, highlighting a higher prevalence with increasing LVEF, a differential clinical profile, similar HF severity, and a non-independent association with mortality in our cohort (Fig. 2). These results show the need for an improved understanding of the AF and HF coexistence phenomenon. Analyzing large HF registries may help elucidate relevant differences in the trends by region.
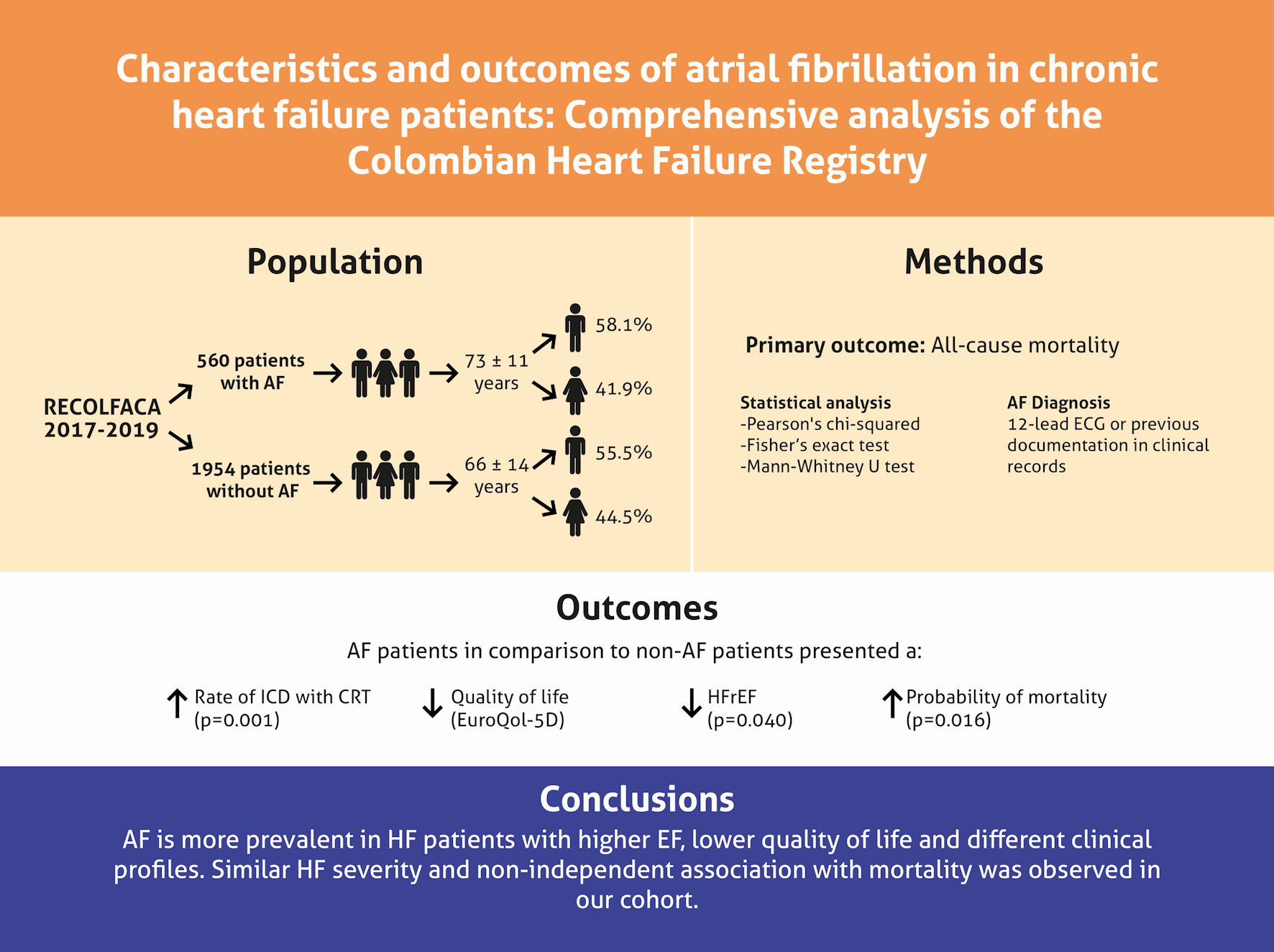 Click for large image | Figure 2. Summary of findings from the RECOLFACA study regarding atrial fibrillation (AF) in chronic HF patients. RECOLFACA: the Colombian Heart Failure Registry; ECG: electrocardiogram; ICD: implantable cardioverter defibrillator; CRT: cardiac resynchronization therapy; HFrEF: heart failure with reduced ejection fraction. |
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they do not have any competing interests.
Informed Consent
Not applicable.
Author Contributions
JEG contributed to the conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, supervision, validation, visualization, writing the original draft and writing, reviewing, and editing of the final version of the manuscript. CS and LEE contributed to conceptualization, investigation, methodology, supervision, writing the original draft, and writing, reviewing, and editing of the final version of the manuscript. ART, JBH, BR, FMJ, JIM, OAP, AHR, PRG, FRT, GTG, MAD and EEC contributed to the investigation and writing, reviewing, and editing of the final version of the manuscript. All authors critically revised and approved the manuscript.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Abbreviations
ACC: American College of Cardiology; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; ARNI: angiotensin receptor neprilysin inhibitor; AF: atrial fibrillation; AHA: American Heart Association; HF: heart failure; HFmrEF: heart failure with mildly reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; ICD: implantable cardioverter defibrillator; LVEF: left ventricle ejection fraction; MRA: mineralocorticoid receptor antagonist; NT-proBNP: N-terminal prohormone of brain natriuretic peptide; NYHA: New York Heart Association; QoL: quality of life; RECOLFACA: Colombian Heart Failure Registry; VALIANT: Valsartan in Acute Myocardial Infarction
| References | ▴Top |
- Caldwell JC, Mamas MA. Heart failure, diastolic dysfunction and atrial fibrillation; mechanistic insight of a complex inter-relationship. Heart Fail Rev. 2012;17(1):27-33.
doi pubmed - Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2-e220.
doi pubmed pmc - Morin DP, Bernard ML, Madias C, Rogers PA, Thihalolipavan S, Estes NA, 3rd. The state of the art: atrial fibrillation epidemiology, prevention, and treatment. Mayo Clin Proc. 2016;91(12):1778-1810.
doi pubmed - Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370-2375.
doi pubmed - Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol. 2014;171(3):368-376.
doi pubmed - Manolis TA, Manolis AA, Apostolopoulos EJ, Melita H, Manolis AS. Atrial fibrillation and cognitive impairment: an associated burden or burden by association? Angiology. 2020;71(6):498-519.
doi pubmed - Ball J, Carrington MJ, McMurray JJ, Stewart S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol. 2013;167(5):1807-1824.
doi pubmed - Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920-2925.
doi pubmed - Lee Park K, Anter E. Atrial Fibrillation and Heart Failure: A Review of the Intersection of Two Cardiac Epidemics. J Atr Fibrillation. 2013;6(1):751.
doi pubmed pmc - Gomez-Mesa JE, Saldarriaga-Giraldo CI, Echeverria LE, Luna-Bonilla P. Grupo Investigador RECOLFACA. Registro colombiano de falla cardiaca (RECOLFACA): resultados. Rev Colomb Cardiol. 2021;28(4):334-342.
- Gomez-Mesa JE, Saldarriaga CI, Echeverria LE, Luna P. RECOLFACA Research Group. Colombian heart failure registry (RECOLFACA): methodology and preliminary data. Rev Colomb Cardiol. 2021;28(3):217-230.
- Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119(18):2516-2525.
doi pubmed - Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32(3):695-703.
doi pubmed - Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91(6A):2D-8D.
doi pubmed - Solti F, Vecsey T, Kekesi V, Juhasz-Nagy A. The effect of atrial dilatation on the genesis of atrial arrhythmias. Cardiovasc Res. 1989;23(10):882-886.
doi pubmed - Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100(1):87-95.
doi pubmed - Middlekauff HR, Stevenson WG, Stevenson LW. Prognostic significance of atrial fibrillation in advanced heart failure. A study of 390 patients. Circulation. 1991;84(1):40-48.
doi pubmed - Carson PE, Johnson GR, Dunkman WB, Fletcher RD, Farrell L, Cohn JN. The influence of atrial fibrillation on prognosis in mild to moderate heart failure. The V-HeFT Studies. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87(6 Suppl):VI102-110.
pubmed - Mahoney P, Kimmel S, DeNofrio D, Wahl P, Loh E. Prognostic significance of atrial fibrillation in patients at a tertiary medical center referred for heart transplantation because of severe heart failure. Am J Cardiol. 1999;83(11):1544-1547.
doi pubmed - Mathew J, Hunsberger S, Fleg J, Mc Sherry F, Williford W, Yusuf S. Incidence, predictive factors, and prognostic significance of supraventricular tachyarrhythmias in congestive heart failure. Chest. 2000;118(4):914-922.
doi pubmed - Crijns HJ, Tjeerdsma G, de Kam PJ, Boomsma F, van Gelder IC, van den Berg MP, van Veldhuisen DJ. Prognostic value of the presence and development of atrial fibrillation in patients with advanced chronic heart failure. Eur Heart J. 2000;21(15):1238-1245.
doi pubmed - Kober L, Swedberg K, McMurray JJ, Pfeffer MA, Velazquez EJ, Diaz R, Maggioni AP, et al. Previously known and newly diagnosed atrial fibrillation: a major risk indicator after a myocardial infarction complicated by heart failure or left ventricular dysfunction. Eur J Heart Fail. 2006;8(6):591-598.
doi pubmed - Swedberg K, Olsson LG, Charlesworth A, Cleland J, Hanrath P, Komajda M, Metra M, et al. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long-term treatment with beta-blockers: results from COMET. Eur Heart J. 2005;26(13):1303-1308.
doi pubmed - Tsuchihashi-Makaya M, Hamaguchi S, Kinugawa S, Yokota T, Goto D, Yokoshiki H, Kato N, et al. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J. 2009;73(10):1893-1900.
doi pubmed - Veenis JF, Brunner-La Rocca HP, Linssen GCM, Smeele FJJ, Wouters N, Westendorp PHM, Rademaker PC, et al. Atrial fibrillation in chronic heart failure patients with reduced ejection fraction: The CHECK-HF registry. Int J Cardiol. 2020;308:60-66.
doi pubmed - Son MK, Park JJ, Lim NK, Kim WH, Choi DJ. Impact of atrial fibrillation in patients with heart failure and reduced, mid-range or preserved ejection fraction. Heart. 2020;106(15):1160-1168.
doi pubmed pmc - Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VP, Parissis J, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19(12):1574-1585.
doi pubmed - Sartipy U, Dahlstrom U, Fu M, Lund LH. Atrial fibrillation in heart failure with preserved, mid-range, and reduced ejection fraction. JACC Heart Fail. 2017;5(8):565-574.
doi pubmed - Lund LH, Claggett B, Liu J, Lam CS, Jhund PS, Rosano GM, Swedberg K, et al. Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail. 2018;20(8):1230-1239.
doi pubmed - Zafrir B, Lund LH, Laroche C, Ruschitzka F, Crespo-Leiro MG, Coats AJS, Anker SD, et al. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: a report from 14 964 patients in the European Society of Cardiology Heart Failure Long-Term Registry. Eur Heart J. 2018;39(48):4277-4284.
doi pubmed - Aronow WS, Ahn C, Kronzon I. Prognosis of congestive heart failure after prior myocardial infarction in older persons with atrial fibrillation versus sinus rhythm. Am J Cardiol. 2001;87(2):224-225.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


