| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 15, Number 3, June 2024, pages 179-188
Effect of Aspirin Use on the Adverse Outcomes in Patients Hospitalized for COVID-19
Poornima Vinoda, b, h, Vinod Krishnappaa, William Rathell Jra, Saira Amirc, Subrina Sundild, Godwin Dogbeye, Hiten Patelf, William Herzogf, g
aDepartment of Internal Medicine, University of North Carolina Health Southeastern, Lumberton, NC, USA
bDepartment of Medicine, Campbell University, Buies Creek, NC, USA
cDepartment of Nephrology, University of Maryland, Baltimore, MD, USA
dDepartment of Nephrology, East Carolina University, Greenville, NC, USA
eCampbell University School of Osteopathic Medicine, Buies Creek, NC, USA
fDepartment of Cardiology, University of North Carolina Health Southeastern, Lumberton, NC, USA
gDepartment of Cardiology, Duke University, Durham, NC, USA
hCorresponding Author: Poornima Vinod, Department of Internal Medicine, Internal Medicine Residency Training, University of North Carolina Health Southeastern, Lumberton, NC 28358, USAand
Manuscript submitted March 31, 2024, accepted May 2, 2024, published online June 25, 2024
Short title: COVID-19 and the Effect of Aspirin
doi: https://doi.org/10.14740/cr1645
| Abstract | ▴Top |
Background: Coronavirus disease 2019 (COVID-19) triggers multiple components of the immune system and causes inflammation of endothelial walls across vascular beds, resulting in respiratory failure, arterial and venous thrombosis, myocardial injury, and multi-organ failure leading to death. Early in the COVID-19 pandemic, aspirin was suggested for the treatment of symptomatic individuals, given its analgesic, antipyretic, anti-inflammatory, anti-thrombotic, and antiviral effects. This study aimed to evaluate the association of aspirin use with various clinical outcomes in patients hospitalized for COVID-19.
Methods: This was a retrospective study involving patients aged ≥ 18 years and hospitalized for COVID-19 from March 2020 to October 2020. Primary outcomes were acute cardiovascular events (ST elevation myocardial infarction (STEMI), type 1 non-ST elevation myocardial infarction (NSTEMI), acute congestive heart failure (CHF), and acute stroke) and death. Secondary outcomes were respiratory failure, need for mechanical ventilation, and acute deep vein thrombosis (DVT)/pulmonary embolism (PE).
Results: Of 376 patients hospitalized for COVID-19, 128 were taking aspirin. Significant proportions of native Americans were hospitalized for COVID-19 in both aspirin (22.7%) and non-aspirin (24.6%) groups. Between aspirin and non-aspirin groups, no significant differences were found with regard to mechanical ventilator support (21.1% vs. 15.3%, P = 0.16), acute cardiovascular events (7.8% vs. 5.2%, P = 0.32), acute DVT/PE (3.9% vs. 5.2%, P = 0.9), readmission rate (13.3% vs. 12.9%, P = 0.91) and mortality (23.4% vs. 20.2%, P = 0.5); however, the median duration of mechanical ventilation was significantly shorter (7 vs. 9 days, P = 0.04) and median length of hospitalization was significantly longer (5.5 vs. 4 days, P = 0.01) in aspirin group compared to non-aspirin group.
Conclusion: No significant differences were found in acute cardiovascular events, acute DVT/PE, mechanical ventilator support, and mortality rate between hospitalized COVID-19 patients who were taking aspirin compared to those not taking aspirin. However, larger studies are required to confirm our findings.
Keywords: Aspirin; Coronavirus disease 2019; Mortality; Hypoxia; Mechanical ventilation; ST-elevation myocardial infarction; Non-ST elevation myocardial infarction; Acute cerebrovascular accidents
| Introduction | ▴Top |
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes coronavirus disease 2019 (COVID-19) affected over 767 million people resulting in over 6.9 million deaths globally [1]. Infection by SARS-CoV-2 triggers multiple components of the immune system, including the innate and adaptive portions as well as antibody response [2]. Based on the current scientific data, SARS-CoV-2 infection is characterized by reduced interferon (IFN)-1 and IFN-3 responses and does trigger multiple pro-inflammatory cytokines, including interleukin (IL)-1B, IL-6, tumor necrosis factor (TNF), and interleukin-1 receptor antagonist (IL-1RA) [2, 3]. These inflammatory molecules are likely to play a significant role in myocardial injury, even though the exact pathophysiology is unknown [4]. Additionally, viral inclusion bodies have been observed under electron microscopy in association with inflammation of endothelial walls across vascular beds of different organs, suggesting the significant role that anti-inflammatory drugs could play in COVID-19 patients [5]. Furthermore, several studies have demonstrated an association of COVID-19 with arterial and venous thrombosis [6-8], even on autopsy [9].
Aspirin is known for its anti-inflammatory function and is used to treat various inflammatory conditions such as Kawasaki disease, pericarditis, and rheumatic fever. The properties of aspirin: anti-inflammatory by inhibition of cyclooxygenase (COX), anti-viral via inhibition of viral replication by inhibiting prostaglandin E2 (PGE2) in macrophages and upregulation of type 1 IFN synthesis, and its anticoagulant effects via inhibition of platelet aggregation, may collectively play an important role in inflammatory cytokine storm and coagulation dysfunction in severe and fatal cases of COVID-19 [10, 11]. Early in the COVID-19 pandemic, aspirin was suggested for the treatment of symptomatic individuals given its analgesic, antipyretic, anti-inflammatory, anti-thrombotic, and antiviral effects, thus combating essentially all aspects of this pathogen simultaneously [12]. In addition to what is known already about the cardioprotective effects of aspirin, Geiger et al described aspirin, as well as its metabolite, salicylic acid, directly reducing SARS-CoV-2 replication possibly via inhibition of nuclear factor kappa-light-chain-enhancer of activated B cells (NFKB) [13]. Aspirin’s immunomodulatory effects have also been demonstrated to work via inhibition of delayed rectifier potassium channels on the surface of T-lymphocytes [14]. This study aimed to evaluate the association of aspirin use with various clinical outcomes, including myocardial injury, acute deep vein thrombosis (DVT)/pulmonary embolism (PE), acute stroke, length of hospitalization, and mortality rate in patients hospitalized for COVID-19.
| Materials and Methods | ▴Top |
This was an observational retrospective study conducted from March 2020 to October 2020 after obtaining approval from the Institutional Review Board (IRB) at the University of North Carolina (UNC) Health Southeastern Hospital. This study was conducted in compliance with the institution’s ethical standards on human subjects, and informed consent was not required. All patients who were aged ≥ 18 years and hospitalized for COVID-19 were included in the study. Data were collected and de-identified by chart review. The variables of interest included patient demographics, comorbid conditions (diabetes mellitus, hypertension, coronary artery disease (CAD), hyperlipidemia, atrial fibrillation, history of DVT or PE, congestive heart failure (CHF), chronic lung disease (CLD), chronic kidney disease (CKD)), home medications, inflammatory markers (D-dimer, lactate dehydrogenase (LDH), ferritin, procalcitonin), chest X-ray findings, inpatient medications, hypoxia (SpO2 < 94%), use of mechanical ventilation, acute medical problems (ST elevation myocardial infarction (STEMI), non-ST elevation myocardial infarction (NSTEMI) type 1, acute CHF, acute cerebrovascular accidents (CVA), acute DVT/PE, acute kidney injury (AKI)), length of hospitalization, readmission, and death.
Primary outcomes were acute cardiovascular events (STEMI, NSTEMI type 1, acute CHF, acute stroke) and death in patients who were taking aspirin compared to those who were not taking them. Secondary outcomes were hypoxia, defined as SpO2 < 94%, need for and length of mechanical ventilation, acute DVT/PE, and readmission rate.
Frequencies/percentages were generated for categorical variables to estimate the rate/proportions of the variables of interest. For continuous variables, measures of central tendency such as mean (or median, as necessary) and dispersion, namely standard deviation (or the range, as appropriate), were computed. Group differences between those taking aspirin vs. those not taking aspirin were explored using Chi-square tests or independent samples t-tests depending on the nature of the outcome of interest - categorical or continuous, respectively. Where appropriate, a Kaplan-Meier (K-M) analysis was conducted. Further, multivariable logistic regression analysis was conducted to account for any confounding factors in the association between aspirin use and the outcomes of interest. All statistical tests were considered significant wherever P ≤ 0.05.
| Results | ▴Top |
Of 376 patients hospitalized for COVID-19 from March 2020 to October 2020, 128 patients were taking aspirin, most of which were females in both aspirin (52.3%) and non-aspirin 59.3% groups (Table 1). The mean age of patients was 66.8 and 59.8 years in the aspirin and non-aspirin groups, respectively. Significant proportions of native Americans were hospitalized for COVID-19 in both aspirin (22.7%) and non-aspirin (24.6%) groups (Table 1). The mean body mass index was 32.71 and 32.4 in the aspirin and non-aspirin groups, respectively (Table 1). A significantly higher proportion of patients had hypoxia in the aspirin group compared to the non-aspirin group (71.1% vs. 54.4%, P = 0.002); however, there was no significant difference in the percentage of people requiring oxygen support via nasal cannula (64.1% vs. 57.3%, P = 0.204), high flow oxygen support (35.2% vs. 28.2%, P = 0.163) and mechanical ventilator support (21.1% vs. 15.3%, P = 0.16) (Table 2). Nonetheless, the median duration of mechanical ventilation was significantly shorter in the aspirin group compared to the non-aspirin group (7 vs. 9 days, P = 0.036) (Table 2). Further, there were no significant differences between aspirin and non-aspirin groups with regard to the levels of inflammatory markers on presentation and at peak (D-dimer, P = 0.91, P = 0.24; LDH, P = 0.93, P = 0.9; ferritin, P = 0.45, P = 0.56; procalcitonin, P = 0.98, P = 0.76) (Table 2). Significantly higher proportions of patients in the aspirin group compared to the non-aspirin group had noticeable chest X-ray changes (82.8% vs. 74.2%, P = 0.06), and were treated with antibiotics (88.3% vs. 79%, P = 0.03), remdesivir (52.3% vs. 33.9%, P = 0.001) and dexamethasone (71.9% vs. 52.4%, P = 0.00) (Table 2). There were no statistically significant differences between the aspirin and the non-aspirin groups with regard to acute cardiovascular events (7.8% vs. 5.2%, P = 0.32) and acute DVT/PE (3.9% vs. 5.2%, P = 0.9); however, a significantly higher percentage of patients in the aspirin group had AKI (44.5% vs. 30.2%, P = 0.006). The median length of hospitalization was significantly longer in the aspirin group compared to the non-aspirin group (5.5 vs. 4 days, P = 0.01); this could reflect the fact that the patients on aspirin were likely sicker compared to the patients not on aspirin (Fig. 1). There was no significant difference in readmission rate (13.3% vs. 12.9%, P = 0.91) and mortality (23.4% vs. 20.2%, P = 0.5) (Table 2).
 Click to view | Table 1. Demographics and Other Baseline Characteristics of COVID-19 Patients Included in the Study |
 Click to view | Table 2. Comparative Analysis of Variables of Interest Between COVID-19 Patients on Aspirin and Those Not on Aspirin |
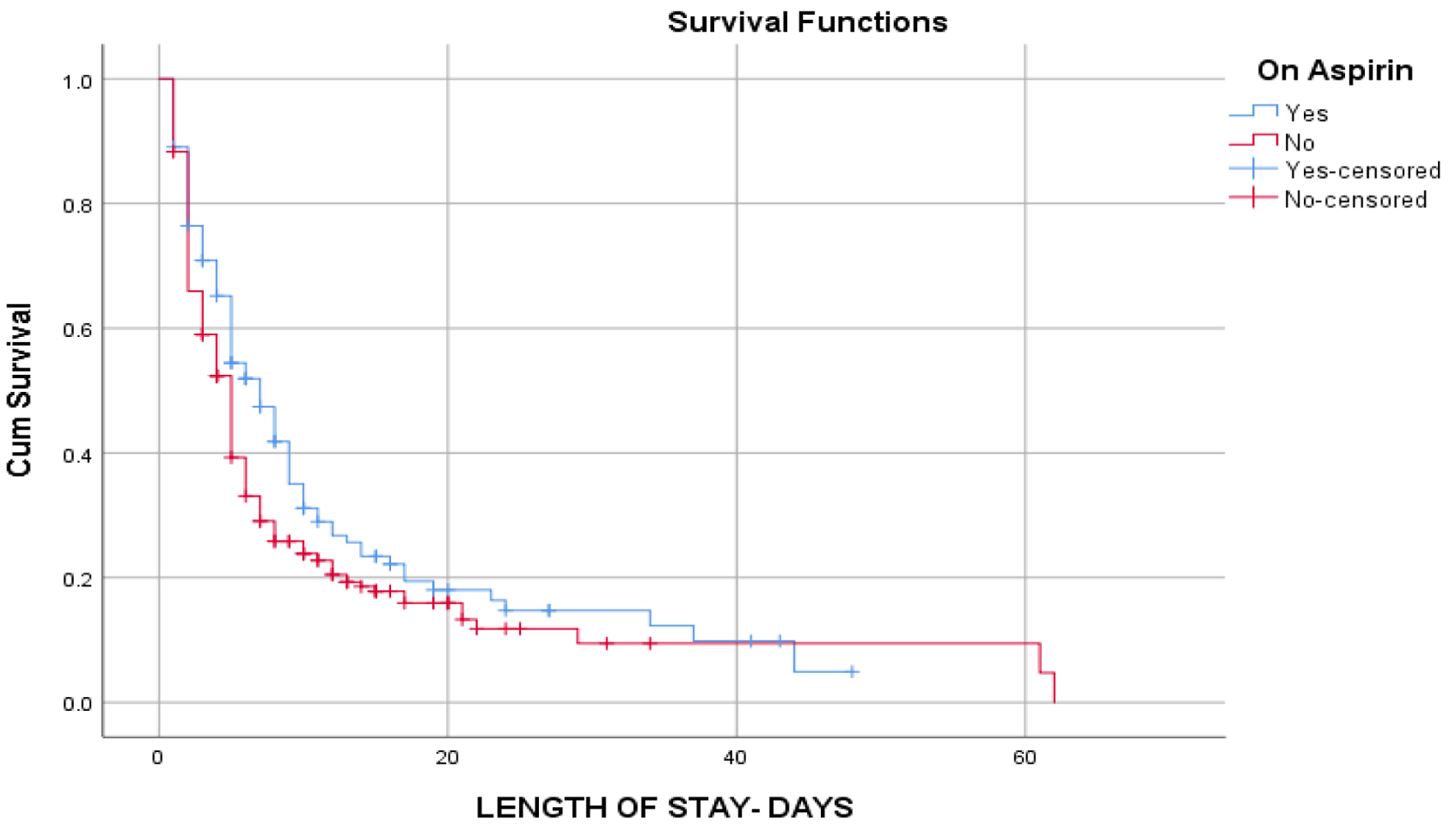 Click for large image | Figure 1. Kaplan-Meier curve showing cumulative survival and length of stay of patients on aspirin compared to patients not on aspirin. Patients on aspirin stayed longer in the hospital compared to those not on aspirin from approximately 5 up to 40 days. |
The effects of age, race, gender, aspirin use, diabetes mellitus, hypertension, CAD, and CKD on the odds of developing hypoxia, requiring mechanical ventilation, acute DVT/PE, readmission rate, and mortality were evaluated using multivariable logistic regression analysis (Tables 3-7). Non-Hispanic Whites and patients younger than 65 years old had significantly higher odds of developing hypoxia (P = 0.049, odds ratio (OR) 2.43, 95% confidence interval (CI) 1.004 - 5.86; P = 0.006, OR 1.95, 95% CI 1.21 - 3.14) respectively (Table 3). Furthermore, the odds of developing acute DVT/PE were significantly higher in male patients (P = 0.011, OR 7.76, 95% CI 1.61 - 37.51) and lower in patients with CKD (P = 0.001, OR 0.15, 95% CI 0.05 - 0.47). Moreover, patients with CKD had lower odds of readmission (P = 0.01, OR 0.4, 95% CI 0.20 - 0.8), and patients aged < 65 years had higher odds of death from COVID-19 (P ≤ 0.00, OR 2.88, 95% CI 1.61 - 5.15). Nonetheless, multivariable logistic regression did not show any association of aspirin use with hypoxia, requirement of mechanical ventilation, acute DVT/PE, readmission, and mortality rates.
 Click to view | Table 3. Multivariable Logistic Regression Analysis for Hypoxia in Hospitalized COVID-19 Patients |
 Click to view | Table 4. Multivariable Logistic Regression Analysis for Mechanical Ventilation in COVID-19 Patients |
 Click to view | Table 5. Multivariable Logistic Regression Analysis for DVT/PE in Hospitalized COVID-19 Patients |
 Click to view | Table 6. Multivariable Logistic Regression Analysis for Readmission in COVID-19 Patients |
 Click to view | Table 7. Multivariable Logistic Regression Analysis for Mortality in COVID-19 Patients |
| Discussion | ▴Top |
Our study did not show statistically significant associations between aspirin use and any primary or secondary outcomes, including acute cardiovascular events, overall mortality, acute DVT/PE, need for mechanical ventilation, or readmission rate. Nonetheless, multiple significant observations were made outside of the primary and secondary outcomes, including a larger number of patients taking aspirin experienced respiratory failure (defined as SpO2 < 94%) and AKI. Prehospital aspirin use has been implicated in decreasing the risk of intensive care unit (ICU) admission and mechanical ventilation without significant effect on mortality [15], and some even suggested the use of troponin and D-dimer levels to guide aspirin therapy [16]. Additionally, the duration of mechanical ventilation was significantly shorter in patients taking aspirin, which could potentially attenuate the endothelial inflammation observed in COVID-19 infection. Furthermore, our study also showed a higher proportion of both men and patients with CKD taking aspirin experienced acute DVT or PE. The exact significance of this remains unclear, although it is well-known that patients with CKD do experience higher levels of platelet dysfunction and hypercoagulability than others [17].
To this point, several studies have evaluated the association between not only aspirin but also non-steroidal anti-inflammatory drugs (NSAIDs) in general, as well as various doses of anticoagulation, and morbidity and mortality associated with COVID-19 infections. One of the most notable of these studies, the RECOVERY trial, a randomized controlled trial (RCT) that assigned COVID-19 patients to receive either aspirin or usual care, demonstrated no reduction in either 28-day mortality or risk of progressing to invasive mechanical ventilation or death but did find a small increase in discharged rate within 28 days [18]. Zhao et al demonstrated with a meta-analysis involving multiple NSAIDs (including aspirin) that NSAIDs use prior to admission or diagnosis of COVID-19 was not associated with an increased rate of hospitalizations, mechanical ventilation, or length of hospital stay; however, their use was, in fact, associated with not only a decreased risk of severe infection and death but also an increase in the risk of stroke [19]. Interestingly, another meta-analysis conducted by Zong et al included 23 observational studies and four RCTs [20]. Analysis of the observational studies did demonstrate lower mortality risk in patients taking antiplatelet medication; however, this was lost after the inclusion of the four RCTs, indicating a lack of benefit in adding these medications to the repertoire of the others used to treat primary COVID-19 infection [20]. Further demonstration of the lack of mortality benefit has also been made elsewhere [21, 22]. One study even showed an increased risk of hospitalization in patients taking aspirin at the time of diagnosis [23].
Numerous studies have demonstrated a pure mortality reduction in COVID-19 patients without other deleterious effects. A meta-analysis performed by Su et al, which included not only retrospective and prospective cohort studies but also four RCTs, noted that specifically, the cohort studies demonstrated mortality reduction, not the RCTs, although significance was not lost after the inclusion of the RCTs within the final analysis [24]. Another study performed by Chow et al also found that aspirin use, defined either as use within 24 h of or in the 7 days prior to hospital admission, was associated with reduced risk for mechanical ventilation, ICU admission, and in-hospital mortality [25]. Furthermore, a multicenter, retrospective study performed by Sisinni et al examined patients already using low-dose aspirin daily for 7 days prior to hospital admission for COVID-19 and found that at 30 days, less number of patients utilizing aspirin prior to admission experienced a composite of in-hospital mortality and/or need for respiratory support upgrade [26]. Similarly, multiple other studies, including meta-analyses and prospective and retrospective observational cohort studies, have demonstrated mortality reduction in COVID-19 patients taking aspirin either prior to admission or after admission [11, 27-30]. In contrast, our study did not demonstrate mortality benefit with the use of aspirin. In line with our results, multiple studies have also demonstrated no link between mortality in patients with COVID-19 and aspirin use [21, 31].
Some studies have investigated the use of not only antiplatelet agents but anticoagulants and immunomodulatory therapies as well. An interesting study by Lima-Morales et al examined the efficacy of the multidrug regimen azithromycin, montelukast, ivermectin, and aspirin in ambulatory COVID-19 patients and found higher rates of recovery and lower rates of hospitalization and death at 14 days after diagnosis [32]. Undoubtedly, however, it is difficult to associate these results with any one drug. Eikelboom et al examined the use of colchicine, representative of one treatment arm, as well as the combination of aspirin and rivaroxaban, the other arm, in symptomatic COVID-19 patients and found no effect on mortality or disease progression in either group [33]. However, a significant number of patients in the aspirin/rivaroxaban group experienced bleeding events [33], further highlighting the need for new trials evaluating not only mortality reduction but also rates of adverse effects, some of which can be, without a doubt, life-threatening.
Given the amount of conflicting information regarding aspirin and other antiplatelet or anticoagulant use in COVID-19 patients existing within the literature, more trials are needed to determine if there is a true association between aspirin use and mortality associated with COVID-19 infection. Additionally, it is evident that these benefits, if and when they are determined, must be carefully weighed against the risks of these medications, such as bleeding, increased need for organ support, or even lapses in efficacy due to, for example, higher levels of thromboxanes in elderly or obese patients, driving resistance to aspirin [34]. Nonetheless, at this time, prescribing aspirin-containing medications to patients diagnosed with COVID-19, whether inside or outside of the hospital, should be a highly individualized process and should involve consideration of previous use, comorbidities, and bleeding propensity.
Strengths and limitations
It is important to note that this study’s limitations include a small sample size and a lack of longer follow-up. This study was done in a community hospital setting with a rural population involving a significant number of native American, Hispanic, and African American participants, which is the greatest strength of this study.
Conclusion
Our study found no significant association between aspirin use and primary or secondary endpoints, including acute cardiovascular events, overall mortality, acute DVT/PE, need for mechanical ventilation, or readmission rate. However, existing literature about aspirin use in COVID-19 patients is highly conflicting, and future large-scale studies are needed to delineate its advantages and disadvantages clearly.
Acknowledgments
None to declare.
Financial Disclosure
This study received no grants from any funding agency.
Conflict of Interest
Authors have no conflict of interest to declare.
Informed Consent
Informed consent was not required.
Author Contributions
Poornima Vinod: writing-original draft, writing and editing, visualization, supervision, resources, project administration, methodology, investigation, data curation, and conceptualization. Vinod Krishnappa: writing-original draft, writing-review and editing, resources, investigation, data curation, and conceptualization. William Rathell: writing-original draft, writing-review and editing, resources, investigation, data curation, and conceptualization. Saira Amir: writing-review and editing, resources, investigation, data curation, and conceptualization. Subrina Sundil: writing-review and editing, resources, investigation, data curation, and conceptualization. Godwin Dogbey: statistical analysis, methodology, investigation, data curation, and conceptualization. Hiten Patel: writing-review and editing, conceptualization, and supervision. William Herzog: writing-review and editing, conceptualization, and supervision.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- WHO COVID-19 dashboard. 2023. Available from: https://covid19.who.int.
- Garcia LF. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2020;11:1441.
doi pubmed pmc - Declercq J, De Leeuw E, Lambrecht BN. Inflammasomes and IL-1 family cytokines in SARS-CoV-2 infection: from prognostic marker to therapeutic agent. Cytokine. 2022;157:155934.
doi pubmed pmc - Babapoor-Farrokhran S, Gill D, Walker J, Rasekhi RT, Bozorgnia B, Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253:117723.
doi pubmed pmc - Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418.
doi pubmed pmc - Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148-150.
doi pubmed pmc - Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-1424.
doi pubmed pmc - Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Muller MCA, Bouman CCS, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995-2002.
doi pubmed pmc - Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24:100434.
doi pubmed pmc - Coulombe F, Jaworska J, Verway M, Tzelepis F, Massoud A, Gillard J, Wong G, et al. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity. 2014;40(4):554-568.
doi pubmed - Wijaya I, Andhika R, Huang I, Purwiga A, Budiman KY. The effects of aspirin on the outcome of COVID-19: a systematic review and meta-analysis. Clin Epidemiol Glob Health. 2021;12:100883.
doi pubmed pmc - Bianconi V, Violi F, Fallarino F, Pignatelli P, Sahebkar A, Pirro M. Is acetylsalicylic acid a safe and potentially useful choice for adult patients with COVID-19? Drugs. 2020;80(14):1383-1396.
doi pubmed pmc - Geiger N, Konig EM, Oberwinkler H, Roll V, Diesendorf V, Fahr S, Obernolte H, et al. Acetylsalicylic acid and salicylic acid inhibit SARS-CoV-2 replication in precision-cut lung slices. Vaccines (Basel). 2022;10(10):1619.
doi pubmed pmc - Kazama I, Senzaki M. Does immunosuppressive property of non-steroidal anti-inflammatory drugs (NSAIDs) reduce COVID-19 vaccine-induced systemic side effects? Drug Discov Ther. 2021;15(5):278-280.
doi pubmed - Sayed Ahmed HA, Merrell E, Ismail M, Joudeh AI, Riley JB, Shawkat A, Habeb H, et al. Rationales and uncertainties for aspirin use in COVID-19: a narrative review. Fam Med Community Health. 2021;9(2):e000741.
doi pubmed pmc - Mohamed-Hussein AAR, Aly KME, Ibrahim MAA. Should aspirin be used for prophylaxis of COVID-19-induced coagulopathy? Med Hypotheses. 2020;144:109975.
doi pubmed pmc - Nunns GR, Moore EE, Chapman MP, Moore HB, Stettler GR, Peltz E, Burlew CC, et al. The hypercoagulability paradox of chronic kidney disease: the role of fibrinogen. Am J Surg. 2017;214(6):1215-1218.
doi pubmed pmc - Group RC. Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399(10320):143-151.
doi pubmed pmc - Zhao H, Huang S, Huang S, Liu F, Shao W, Mei K, Ma J, et al. Prevalence of NSAID use among people with COVID-19 and the association with COVID-19-related outcomes: systematic review and meta-analysis. Br J Clin Pharmacol. 2022;88(12):5113-5127.
doi pubmed pmc - Zong X, Wang X, Liu Y, Li Z, Wang W, Wei D, Chen Z. Antiplatelet therapy for patients with COVID-19: systematic review and meta-analysis of observational studies and randomized controlled trials. Front Med (Lausanne). 2022;9:965790.
doi pubmed pmc - Yuan S, Chen P, Li H, Chen C, Wang F, Wang DW. Mortality and pre-hospitalization use of low-dose aspirin in COVID-19 patients with coronary artery disease. J Cell Mol Med. 2021;25(2):1263-1273.
doi pubmed pmc - Connors JM, Brooks MM, Sciurba FC, Krishnan JA, Bledsoe JR, Kindzelski A, Baucom AL, et al. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial. JAMA. 2021;326(17):1703-1712.
doi pubmed pmc - Morrison FJ, Su M, Turchin A. COVID-19 outcomes in patients taking cardioprotective medications. PLoS One. 2022;17(10):e0275787.
doi pubmed pmc - Su W, Miao H, Guo Z, Chen Q, Huang T, Ding R. Associations between the use of aspirin or other antiplatelet drugs and all-cause mortality among patients with COVID-19: a meta-analysis. Front Pharmacol. 2022;13:989903.
doi pubmed pmc - Chow JH, Khanna AK, Kethireddy S, Yamane D, Levine A, Jackson AM, McCurdy MT, et al. Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019. Anesth Analg. 2021;132(4):930-941.
doi pubmed - Sisinni A, Rossi L, Battista A, Poletti E, Battista F, Battista RA, Malagoli A, et al. Pre-admission acetylsalicylic acid therapy and impact on in-hospital outcome in COVID-19 patients: the ASA-CARE study. Int J Cardiol. 2021;344:240-245.
doi pubmed pmc - Srinivasan A, Brown J, Krishnamani PP, Cornett B, Kesavan RB, Sarva ST, Raza SA, et al. Aspirin use is associated with decreased inpatient mortality in patients with COVID-19: a meta-analysis. Am Heart J Plus. 2022;20:100191.
doi pubmed pmc - Martha JW, Pranata R, Lim MA, Wibowo A, Akbar MR. Active prescription of low-dose aspirin during or prior to hospitalization and mortality in COVID-19: a systematic review and meta-analysis of adjusted effect estimates. Int J Infect Dis. 2021;108:6-12.
doi pubmed pmc - Lal A, Garces JPD, Bansal V, Tekin A, Zec S, Khanna AK, Warner MA, et al. Pre-hospital aspirin use and patient outcomes in COVID-19: results from the International Viral Infection and Respiratory Illness Universal Study (VIRUS). Arch Bronconeumol. 2022;58(11):746-753.
doi pubmed pmc - Meizlish ML, Goshua G, Liu Y, Fine R, Amin K, Chang E, DeFilippo N, et al. Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: a propensity score-matched analysis. Am J Hematol. 2021;96(4):471-479.
doi pubmed pmc - Lund LC, Kristensen KB, Reilev M, Christensen S, Thomsen RW, Christiansen CF, Stovring H, et al. Adverse outcomes and mortality in users of non-steroidal anti-inflammatory drugs who tested positive for SARS-CoV-2: a Danish nationwide cohort study. PLoS Med. 2020;17(9):e1003308.
doi pubmed pmc - Lima-Morales R, Mendez-Hernandez P, Flores YN, Osorno-Romero P, Sancho-Hernandez CR, Cuecuecha-Rugerio E, Nava-Zamora A, et al. Effectiveness of a multidrug therapy consisting of ivermectin, azithromycin, montelukast, and acetylsalicylic acid to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico. Int J Infect Dis. 2021;105:598-605.
doi pubmed pmc - Eikelboom JW, Jolly SS, Belley-Cote EP, Whitlock RP, Rangarajan S, Xu L, Heenan L, et al. Colchicine and the combination of rivaroxaban and aspirin in patients hospitalised with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial. Lancet Respir Med. 2022;10(12):1169-1177.
doi pubmed pmc - Chiang KC, Gupta A. Aspirin resistance in obese and elderly patients with COVID-19? Am J Med. 2021;134(4):e297.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


