| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 15, Number 4, August 2024, pages 281-297
Efficacy of Beta-Blockers and Angiotensin-Converting Enzyme Inhibitors in Non-Ischemic Dilated Cardiomyopathy: A Systematic Review and Meta-Analysis
Jordan Llerena-Velasteguia, b, f , Melisa Santamaria-Lassoa
, Melany Mejia-Moraa
, Mauricio Santander-Aldeana
, Andrea Granda-Munoza
, Claudia Hurtado-Alzatec
, Ana Clara Fonseca Souza de Jesusd
, Jurgen Baldelomar-Ortize
aMedical School, Pontifical Catholic University of Ecuador, Quito, Ecuador
bResearch Center, Center for Health Research in Latin America (CISeAL), Quito, Ecuador
cMedical School, University of La Sabana, Cundinamarca, Colombia
dMedical School, Faculdade de Minas - FAMINAS-BH, Belo Horizonte, Brazil
eMedical School, Private University of the Valley, La Paz, Bolivia
fCorresponding Author: Jordan Llerena-Velastegui, Medical School, Pontifical Catholic University of Ecuador, Quito, Ecuador
Manuscript submitted May 8, 2024, accepted June 10, 2024, published online July 18, 2024
Short title: Beta-Blockers and ACEI in NIDCM
doi: https://doi.org/10.14740/cr1653
| Abstract | ▴Top |
Background: Non-ischemic dilated cardiomyopathy (NIDCM) is a form of heart failure with a poor prognosis and unclear optimal management. The aim of the study was to systematically review the literature and assess the efficacy and safety of beta-blockers and angiotensin-converting enzyme (ACE) inhibitors in the management of chronic heart failure secondary to NIDCM and explore their putative mechanisms of action.
Methods: Studies from 1990 to 2023 were reviewed using PubMed and EMBASE, focusing on their effects on left ventricular ejection fraction (LVEF) in NIDCM patients, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results: Beta-blockers showed a significant beneficial effect on LVEF improvement in NIDCM, with an overall effect size of Cohen’s d = 1.30, 95% confidence interval (CI) (0.76, 1.84), high heterogeneity (Tau2 = 0.90; Chi2 = 162.05, df = 13, P < 0.00001; I2 = 92%), and a significant overall effect (Z = 4.72, P < 0.00001). ACE inhibitors also showed a beneficial role, but with less heterogeneity (Tau2 = 0.02; Chi2 = 1.09, df = 1, P = 0.30; I2 = 8%) and a nonsignificant overall effect (Z = 1.36, P = 0.17), 95% CI (-0.24, 1.31).
Conclusions: The study highlights the efficacy of carvedilol in improving LVEF in NIDCM patients over ACE inhibitors, recommends beta-blockers as first-line therapy, and advocates further research on ACE inhibitors.
Keywords: Non-ischemic dilated cardiomyopathy; Beta-blockers; ACE inhibitors; Left ventricular ejection fraction; Systematic review; Meta-analysis
| Introduction | ▴Top |
Dilated cardiomyopathies are one of the leading causes of increased healthcare burden in the field of cardiology, and non-ischemic dilated cardiomyopathy (NIDCM) has been identified as the primary cause of heart failure (HF) and sudden cardiac death [1]. NIDCM is defined through genetic molecular studies and the World Health Organization (WHO) as a force generation disease arising from cytoskeletal abnormalities. It is characterized by “left ventricular (LV) enlargement and global systolic function impairment, specifically a left ventricular ejection fraction (LVEF) of less than 45%, in the absence of coronary artery disease (CAD) or increased loading conditions (hypertension, valve disease)” [2]. This definition was consistently applied as an inclusion criterion in all the included trials in our meta-analysis to ensure uniformity and relevance.
Primarily a muscular disease of the heart, NIDCM (also known as idiopathic dilated cardiomyopathy) has a poor prognosis, with tertiary referral centers observing the death of a staggering 50% of patients within 2 years [3]. This dismal prognosis, however, has shown improvement owing to faster formulation of diagnosis and better management through pharmacokinetic drugs, including multifactorial approaches of vasodilators, antiarrhythmic, and anticoagulation drugs [4]. Despite advances in diagnosis and management, the optimal treatment for NIDCM remains unclear. Currently, guidelines often extrapolate treatment approaches from other types of cardiomyopathies, leaving a gap in specific evidence for NIDCM.
In particular, the comparative efficacy of beta-blockers and angiotensin-converting enzyme (ACE) inhibitors in improving outcomes for NIDCM patients is not well established. The objective of this study was to systematically review and assess the efficacy and safety of beta-blockers and ACE inhibitors in managing chronic HF secondary to NIDCM, aiming to provide clearer guidance for clinical practice.
Pathophysiology of the heart in case of NIDCM
To understand the reason for such sudden developments causing a poorer prognosis, the pathophysiology of NIDCM must be comprehended. Complex structural and functional changes in the heart muscle that impair its pumping capacity have been implicated in the diminished prognosis of NIDCM. To summarize, the following key pathological features help us understand the diverse phenomena contributing to the prognosis and symptoms of NIDCM:
Myocardial remodeling
Due to acute and chronic stressors, there is remodeling and dilation of the left ventricle, accompanied by thinning of the ventricular walls [5]. This impairs contractility and the heart’s pumping action.
Myocyte cellular changes
Individual cardiomyocytes display abnormalities in structure, signaling pathways, calcium handling, energy production, gene expression, etc. [6]. These changes impair excitation-contraction coupling and contractile force generation.
Neurohormonal activation
To compensate for reduced cardiac output, sympathetic activation and the release of hormones like norepinephrine initially occur [7]. However, chronic exposure leads to further deterioration of myocardial structure and function.
Inflammation
Upregulation of inflammatory mediators like tumor necrosis factor (TNF)-alpha, interleukin (IL)-1B, IL-6, and immune cell infiltration can worsen myocardial injury in NIDCM [8].
Genetics
Around 20-35% of cases have gene mutations affecting proteins integral to myocardial cell structure/function, which can include sarcomere protein mutations like those encoding β-cardiac myosin heavy chain (MYH7), cardiac myosin-binding protein-C (MYBPC3), cardiac troponin T (TNNT2), cardiac troponin I (TNNI3), essential myosin light chain (MYL3), regulatory myosin light chain (MYL2), α-tropomyosin (TPM1), cardiac actin (ACTC), and titin (TTN) [9].
Beta-blockers and mechanism of action
Beta-blockers have been hailed as a significant advancement in the field of cardiology since the discovery of digoxin in the 19th century. Naturally, they play a profound role in the management of cardiomyopathies of all kinds, especially NIDCM. As research uncovers precise mechanisms in subtypes like NIDCM, optimized beta-blocker regimens may offer further gains. The potential benefits of beta-blockers could be due to countering the pathological progression of the disease mentioned above. For instance, beta-blockers have shown a role in the downregulation of cardiac remodeling [10].
A key factor is attenuating the exaggerated neurohormonal cascade central to NIDCM pathophysiology [11]. By antagonizing elevated circulating catecholamines, beta-blockers can dampen resultant norepinephrine-induced impairments in cardiomyocyte structure and function. This, in turn, also impacts blood pressure, heart rate, and the workload of myocytes, breaking a cycle of escalating myocardial decompensation [11]. Beta blockade helps prevent adverse left ventricular remodeling through favorably altering genes governing hypertrophy, fibrosis, and myocardial stiffness [12]. By mitigating such cardinal molecular and histopathological disease features, beta-blockers may beneficially modify NIDCM’s natural progression.
ACE inhibitors and mechanism of action
Although they are a relatively new addition to the regimen for NIDCM, ACE inhibitors have been favored due to their proposed mechanism of action, which promises to deliver significant advantages to the living standards of patients. The main mechanisms of action of ACE inhibitors in the management of NIDCM are related to countering ongoing maladaptive neurohormonal activation and preventing adverse cardiac remodeling: 1) Blocking the conversion of angiotensin I to the vasoconstrictor angiotensin II reduces overall activation of the renin-angiotensin-aldosterone system (RAAS). This pathway is important in contributing to disease progression and myocardial damage in NIDCM through cell death promotion, extracellular matrix deposition, and fibrogenesis [13]. 2) Similarly, reducing levels of angiotensin II is thought to lower aldosterone secretion, alleviating sodium retention and edema formation that can further stress the failing heart in NIDCM [14]. 3) ACE inhibitors can inhibit the breakdown of bradykinin, allowing this vasodilator, antifibrotic, and cardioprotective agent to accumulate and help prevent maladaptive cardiac remodeling in NIDCM [15]. 4) By directly counteracting vasoconstrictor, inflammatory, proliferative, fibrotic, and cell death responses resulting from excess RAAS activity, ACE inhibitors attenuate key circulatory, structural, and functional abnormalities central to NIDCM’s pathophysiology [16].
Objectives
The therapeutic efficacy and safety profile of beta-blockers and ACE inhibitors in the management of chronic HF secondary to NIDCM remain unclear. Therefore, in this systematic review and meta-analysis, we aim to 1) Assess efficacy: The efficacy of beta-blocker and ACE inhibitor treatment in NIDCM patients will be evaluated by analyzing pooled relative risks from qualifying randomized trials reporting all-cause and cardiovascular mortality, frequency of hospitalizations, major adverse cardiovascular events, and HF symptom scale scores before and after therapy. 2) Evaluate safety: To ascertain the safety of beta-blockers and ACE inhibitors in this population, we will synthesize data on the overall quality of life (QoL) using validated assessment tools, the development of adverse reactions, treatment withdrawals due to side effects, and patient prognosis concerning transplantation-free survival from the available literature.
Rationale
Currently, small trials have indicated some benefit of standard HF therapies, such as beta-blockers and ACE inhibitors, in NIDCM patients. However, their efficacy in this specific population remains uncertain. While these agents are strongly recommended for reducing adverse outcomes in patients with reduced ejection fraction HF from ischemic etiologies, their precise effects in NIDCM remain ambiguous. For instance, patients with NIDCM display distinct pathophysiological phenotypes like higher levels of inflammatory biomarkers, greater cardiomyocyte apoptosis, and inferior responses to cardiac resynchronization compared to ischemic cardiomyopathy groups. Hence, the effectiveness demonstrated in other cohorts may not translate to NIDCM [17, 18].
Furthermore, current guidelines do not provide specific recommendations for managing NIDCM and simply extrapolate treatment approaches from other cardiomyopathies. This assumption of equivalence in therapeutic responsiveness, however, warrants further verification. Given NIDCM’s distinct phenotypic profile, it is crucial to ascertain the efficacy of standard agents specifically in this high-risk population [19, 20].
Therefore, our systematic review aims to address this knowledge gap by compiling available clinical trial data on beta-blocker and ACE inhibitor treatment in NIDCM cohorts. Our findings will help determine if these agents confer a mortality/morbidity benefit and inform the development of tailored management strategies for NIDCM patients. This could optimize outcomes for this understudied group that bears a substantial HF disease burden.
| Materials and Methods | ▴Top |
The Institutional Review Board approval is not applicable, as this study does not involve primary data collection from human subjects. This review has been successfully registered in the international database PROSPERO, under the ID CRD42024524619. The need for an ethics statement is not applicable as this study is based solely on previously published literature.
Eligibility criteria
We established the eligibility criteria for studies following the Population, Intervention, Comparison, Outcome, and Study Design (PICOS) scheme, as recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The methodological rigor underpinning this review is exemplary, evident from the meticulous approach employed in information retrieval to the systematic organization of the review’s structure. The description of the search process provides a clear roadmap, offering transparency and ensuring that no stone is left unturned in the quest for relevant data. Moreover, the elucidation of the statistical methods utilized to analyze the wealth of gathered information instills confidence in the reliability and validity of the findings.
The following inclusion criteria were used: 1) Studies conducted between 1990 and 2023; 2) Studies with abstracts and/or free full-texts available were selected; 3) Studies that provided pre-protocol and post-protocol test scores for analysis; 4) Studies in the English language; 5) Studies with placebo groups; 6) Studies discussing the impact on QoL post-treatment.
The following exclusion criteria were used: 1) Studies older than 1990; 2) Studies in a foreign language; 3) Studies without abstracts or full texts; 4) Studies missing either pre-protocol or post-protocol test scores; 5) Studies without placebo groups; 6) Studies with a high risk of bias, as identified by the Cochrane risk-of-bias tool available online; 7) Studies with outcomes irrelevant to our measurable variables.
Given the rare and complex nature of NIDCM, developing robust yet specific eligibility criteria is crucial. We included only contemporary studies from the last 30 years (1990 - 2023) to capture current diagnostic and therapeutic practices for this evolving disease. Additionally, we required English language studies as well as access to abstracts and full texts to thoroughly assess relevance and study quality.
Pre- and post-intervention efficacy data are vital to quantify treatment effects, hence requiring these was necessary. Placebo-controlled studies allow differentiation of true treatment effects from confounders like natural disease fluctuation and placebo effect. Assessing QoL is essential to fully evaluate patient-centered outcomes in a profoundly life-altering illness like NIDCM [21, 22].
We leveraged large databases like PubMed, EMBASE, and Cochrane Central to capture the maximum relevant literature on this rare disease. Moreover, we hand-searched cardiology conference abstracts from 1990 to 2023 to include the latest unpublished trial data on novel and emerging NIDCM therapies. Conference proceedings often house cutting-edge initial trial findings yet to reach journal publication. Further, we searched the clinical trials registry to identify completed but unpublished NIDCM trials to combat publication bias towards positive studies and ensure the inclusion of all pertinent efficacy data.
We excluded pre-1990 studies to focus on modern NIDCM patients who benefit from contemporary innovations in medical therapies, device-based treatments, advanced diagnostics, and HF management programs - all of which profoundly affect outcomes. Our stringent study quality assessment using the Cochrane risk-of-bias tool also selected weaker studies prone to bias, which could skew results.
Information sources
We combed several digitally available databases for relevant literature. These included PubMed, Google Scholar, Elsevier, Science Direct, BMJ, Medline, and journals related to the topic of interest. Prime sources of literature for this study were the Journal of the American Heart Association (JAHA), the European Society of Cardiology Journal (ESC), and others.
Search strategy
In our systematic review and meta-analysis, we rigorously identified and analyzed 30 studies, encompassing a total of 9,190 participants, which met our comprehensive inclusion criteria. The methodology behind our search was extensive, focusing on capturing a wide array of relevant studies within our research scope. To ensure the selection of high-quality and pertinent research, we employed a range of filters and scrutinized various databases, aligning our approach with the specific objectives of our study.
Additionally, we extended our search to include an in-depth examination of the reference lists of the selected studies. This step was crucial in uncovering additional literature, thereby enriching the authenticity and depth of our background knowledge for the systematic review and meta-analysis.
Detailed exploration of our search strategy, including the specific criteria and methodologies employed, is shown here (Supplementary Material 1, www.cardiologyres.org). This document provides a comprehensive overview of our systematic and thorough approach, highlighting our commitment to a rigorous and expansive review of the literature in our field of study.
Selection process
To identify relevant studies for inclusion, we conducted a systematic search of the PubMed, EMBASE, and Cochrane databases in accordance with the PRISMA guidelines. Three reviewers carried out the search, focusing on peer-reviewed randomized controlled trials (RCTs), retrospective cohorts, and longitudinal studies published in English. These studies evaluated beta-blockers and/or ACE inhibitors in adult patients with NIDCM. Two researchers independently screened titles, abstracts, and full texts against pre-specified criteria. These criteria included a population with NIDCM in sinus rhythm, intervention with beta-blocker or ACE inhibitor therapy, a comparator group receiving placebo or standard care, and outcomes of interest such as mortality, hospitalization, HF symptoms, and adverse events. Disagreements regarding study inclusion were resolved through consensus among all three reviewers after re-evaluating the disputed article.
Exclusion reasons were articulated before removing a study from the review. Studies were excluded for various reasons: 1) Issues with the population; 2) Study design not suitable for our analysis; 3) Measurement of irrelevant outcomes; 4) High risk of bias; and 5) Inadequate data for our primary outcome. Sometimes, multiple reasons combined to justify exclusion.
After removing duplicates, initial database searches yielded 1,050 records. Screening excluded 8,110 articles, primarily due to ineligible study designs or interventions, unmatched disease populations, or lack of relevant outcomes. This resulted in 16 unique randomized trials and cohorts enrolling a total of 954 NIDCM patients eligible for qualitative and quantitative analysis, as described below. The use of multiple reviewers during structured screening phases helped minimize the risk of subjective bias in study selection for this systematic review.
Data items
Our predefined systematic search strategy identified 9,190 potential studies for screening. The application of previously outlined eligibility criteria resulted in the selection of 16 randomized placebo-controlled trials and cohorts suitable for inclusion (Fig. 1). The included studies, sample sizes, interventions, comparators, and primary endpoints are summarized in Figure 1.
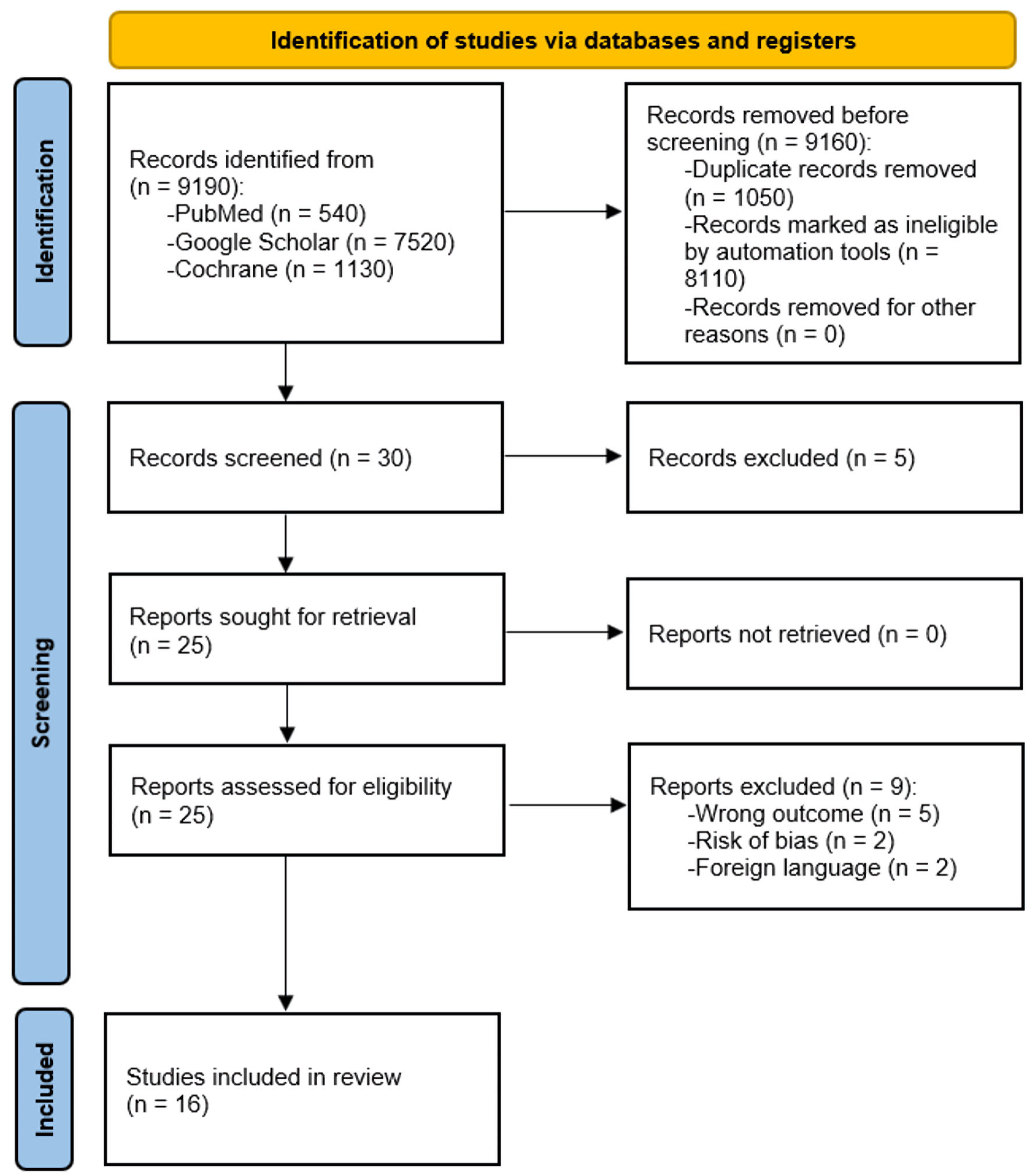 Click for large image | Figure 1. PRISMA flowchart illustrating the study selection process. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
To appraise study quality and minimize bias risk, we employed various methods consistent with Cochrane and PRISMA recommendations [17]. These methods included restricting literature sources to peer-reviewed journals, establishing strict a priori study inclusion/exclusion criteria, having multiple independent reviewers assess eligibility, and excluding prior pooled analyses or literature reviews. Two researchers independently evaluated each included trial for sources of bias using the Cochrane risk-of-bias tool. This evaluation covered domains such as random sequence generation, allocation concealment, blinding of participants/personnel, completeness of outcome data, selective reporting, and other biases through Critical Appraisal Skills Programmer (CASP) analysis. Individual study ratings and composite quality assessments are detailed in Table 1 [20, 23-25].
 Click to view | Table 1. Quality Assessment Using the CASP Tool |
These measures systematically addressed potential heterogeneity and biases across included trials. Limiting evidence sources strictly to high-quality RCTs reporting protocol-specified clinical outcomes also reduced the likelihood of selective reporting or publication bias influencing observed effect measures. Rigorous critical appraisal and synthesis provide confidence in the validity of our qualitative and quantitative conclusions.
After completing the study selection process, we tabulated the study interventions against the study population and the outcomes studied, mentioning only the relevant themes of the outcome in the synthesis table. Bias minimization was achieved by 1) selecting high-quality research and conducting a thorough literature review; 2) eliminating double standards concerning peer review and informed consent in clinical research and practice; and 3) requiring peer reviewers to declare conflicts of interest. To maintain study standards, systematic and narrative reviews were often excluded from the literature. These guidelines detect and eliminate bias in the study protocol, in accordance with Chalmers et al (1990) [17], and their stages of removing publication bias.
Study of risk of bias assessment
All studies selected for quality assessment were analyzed for publication bias. Each study was manually checked for intervention characteristics, population demographics, and outcomes domains. Studies eligible for analysis were independently selected based on the Cochrane criteria for risk of bias. We calculated the risk of bias using the Cochrane risk-of-bias (version 2019) online tool (Higgins et al, 2011) [18]. According to the Cochrane protocol, the risk of bias algorithm assessed five domains of potential risk of bias. These domains were as follows: 1) Bias due to the randomization process; 2) Deviation from intended intervention; 3) Missing outcome data; 4) Measurement of the outcome; 5) Selection of the reported result. Two researchers decoded all relevant data for the purpose of risk assessment.
Synthesis methods
For the 12 randomized trials meeting eligibility criteria, two independent reviewers used standardized forms to extract and tabulate relevant data. The data included study design, patient demographics, details of interventions and comparators, prespecified primary and secondary outcomes, follow-up duration, and results, including effect sizes and measures of variability. Any discrepancies were resolved through discussion and review of the original articles. The four cohorts underwent CASP assessment.
Primary outcomes of interest were changes in LVEF, QoL measures, and serious treatment-related adverse events. Secondary outcomes included all-cause mortality, cardiovascular mortality, frequency of hospitalizations, and composite major adverse cardiac events. Subgroup analyses grouped trials by specific medication class and duration. All analyses were performed using Review Manager software (RevMan) (computer program), version 5.4, The Cochrane Collaboration, 2020, with P < 0.05 defined as statistically significant.
Meta-analyses used random-effects models to pool risk ratios for dichotomous outcomes and weighted mean differences (MD) for continuous variables across studies. Heterogeneity was assessed using the I2 statistic and χ2 test. Sensitivity analyses, excluding studies with high dropout rates or unclear/high sources of bias, were performed to evaluate the consistency and precision of results.
By systematically compiling data and applying meta-analytic techniques, we aimed to provide precise effect calculations concerning the risks, benefits, and therapeutic efficacy of beta-blocker and ACE inhibitor treatment specifically for patients with NIDCM.
| Results | ▴Top |
Study characteristics
In the final sample, 16 studies were manually selected, comprising 12 randomized control trials and four cohort studies. The sample sizes of the populations in each study ranged from 14 to 383. Follow-up periods varied from 2 months to 24 months (2 years). The results of the systematic review revealed that 100% (16/16) of the studies advocated the effectiveness of beta-blockers and ACE inhibitors as essential for patients with HF due to NIDCM. Among specific beta-blocker agents, carvedilol was given higher status over metoprolol due to its better outcomes, even in treatment-resistant patients. ACE inhibitors were found to enhance exercise tolerance, improve lifestyle, and improve the hemodynamic metrics of patients. The synthesis for the systematic review is summarized in Table 2 [19-34].
 Click to view | Table 2. Synthesis Table of Systematic Review Results |
Risk of bias
As previously indicated, each study incorporated into the meta-analysis underwent a risk of bias evaluation. Ultimately, the final sample consisted only of studies that demonstrated a “low” risk of bias across all domains. For the final evaluation, a “traffic lights” plot was created using the Cochrane risk-of-bias tool. Figure 2 displays the risk of bias plot for the 12 primary randomized control trial studies.
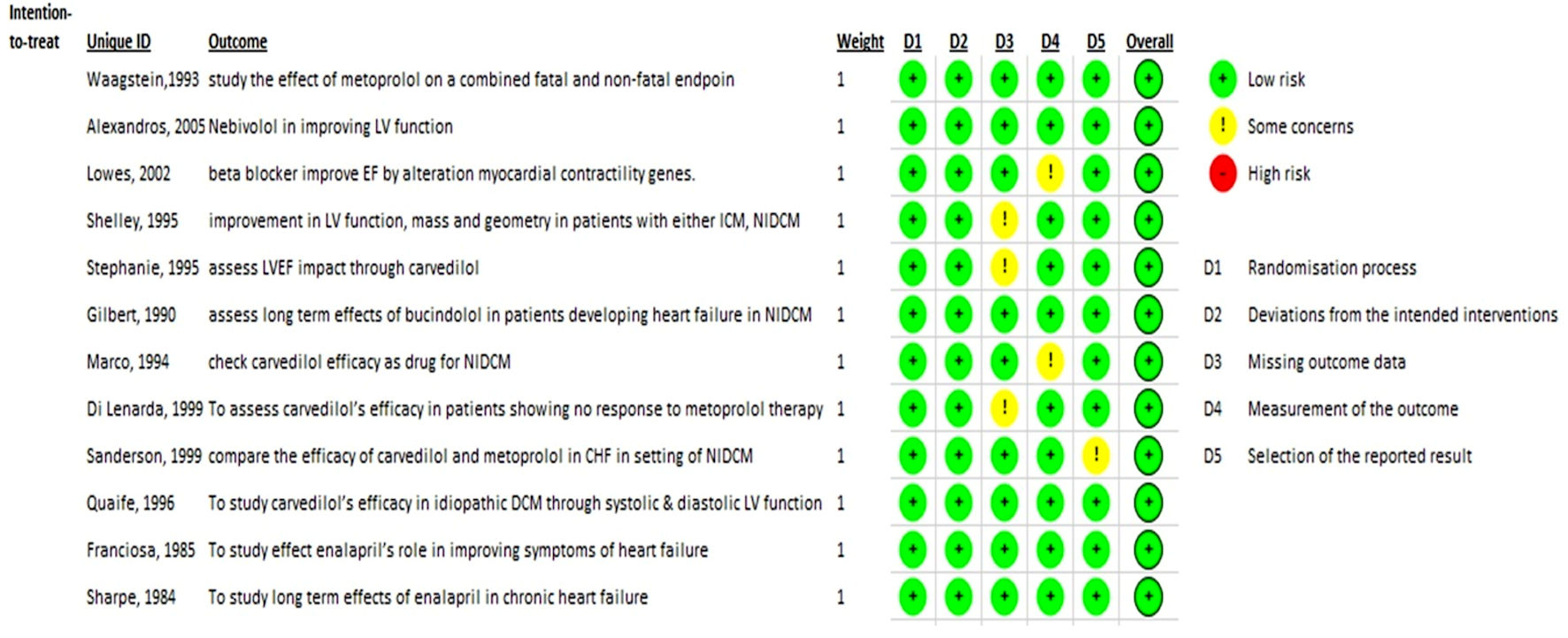 Click for large image | Figure 2. Cochrane risk-of-bias (ROB) traffic light plot. |
Forest plot
Forest plot for beta-blockers
A forest plot for 16 individual studies was created to analyze continuous data. We selected a random-effects model to calculate the deviation and differences in the mean (M) and standard deviation (SD) using the standardized mean difference (SMD) scale. The 95% confidence interval (CI) was plotted on the horizontal axis, with the “point estimation” represented by green squares on the plot. The total sample size (n = 383, 14, 60, 49, 30, 78, 16, 51, 60, 24, 40, 30, 23, 36) remained relatively consistent in the control groups. The central vertical line indicates a state of “no effect”. This forest plot summarized quantitative data about each study and provided an estimated overall quantitative value for all the combined effects. The overall effect size was calculated in terms of Cohen’s d, which was found to be d = 1.30, 95%CI: 0.76 - 1.84. The individual effect size was significant for 13 out of 14 studies. Heterogeneity was calculated as follows: Tau2 = 0.90; Chi2 = 162.05, df = 13 (P < 0.00001); I2 = 92%. The analysis for the overall effect was Z = 4.72 (P < 0.00001). The individual effects of all the studies favored the experimental group, i.e., the population receiving beta-blockers [19]. The MD was -0.53, with 95% CI: (-1.24, 0.18) for the study conducted by Seghatol et al (2004) [20], which was the only study not favoring the experimental group. Findings are summarized in Figure 3.
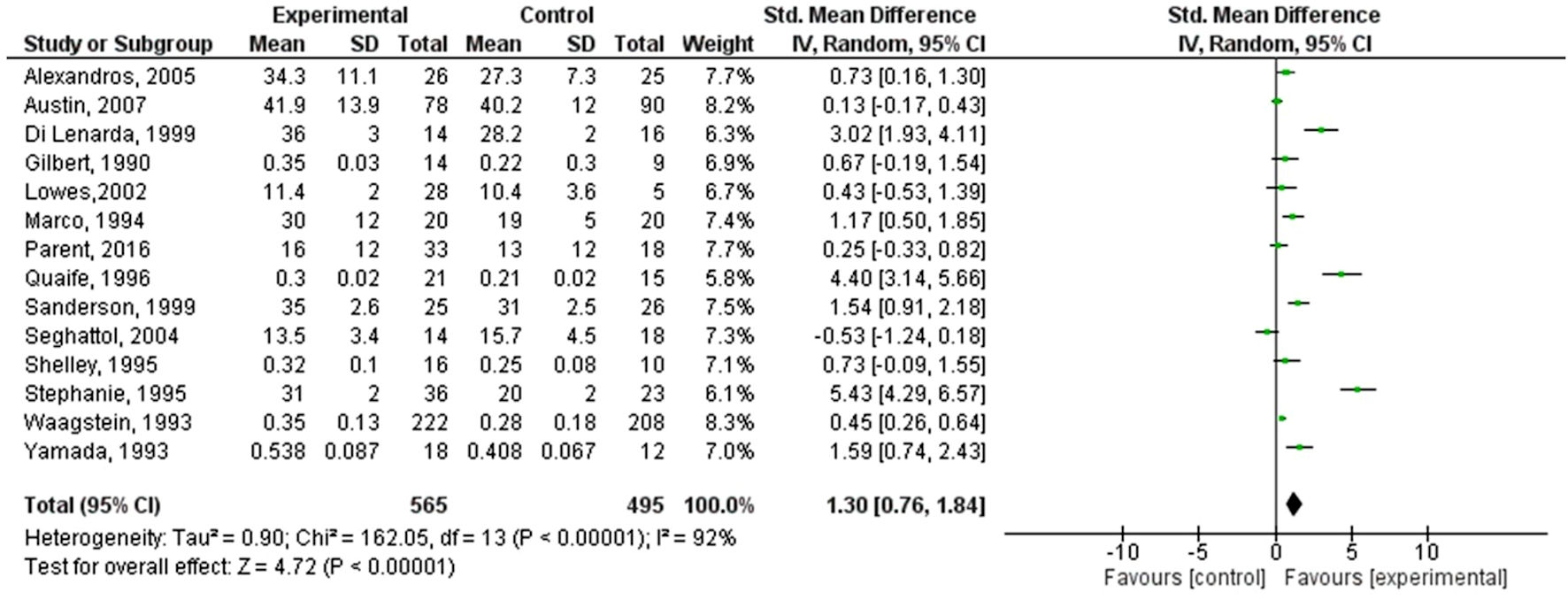 Click for large image | Figure 3. Forest plot of the improvement in left ventricular ejection fraction with beta-blockers. SD: standard deviation; CI: confidence interval. |
Forest plot for ACE inhibitors
A forest plot for two individual studies was created to analyze continuous data. We utilized a random-effects model to calculate the deviation and differences in the M and SD using the SMD scale. The 95% CI was plotted on the horizontal axis, with the “point estimation” represented by green squares on the plot. The total sample size (n = 13, 28) remained relatively consistent in the control groups. The central vertical line indicates a state of “no effect”. This forest plot summarized quantitative data about each study and provided an estimated overall quantitative value for all the combined effects. The overall effect size was calculated in terms of Cohen’s d, which was found to be d = 0.54, 95% CI: (-0.24, 1.31). The individual effect size was significant for both studies. Heterogeneity was calculated as follows: Tau2 = 0.02; Chi2 = 1.09, df = 1 (P = 0.30); I2 = 8%. The analysis for the overall effect was Z = 1.36 (P = 0.17). The individual effects of all the studies favored the experimental group, i.e., the population receiving ACE inhibitors. The MD was 0.11, with 95% CI: (-0.98, 1.20) for the study conducted by Franciosa et al (1985) [33], whereas the MD was -4.19 with a CI of 95% (-8.22, -0.16) for Sharpe et al (1984) [34]. Findings are summarized in Figure 4.
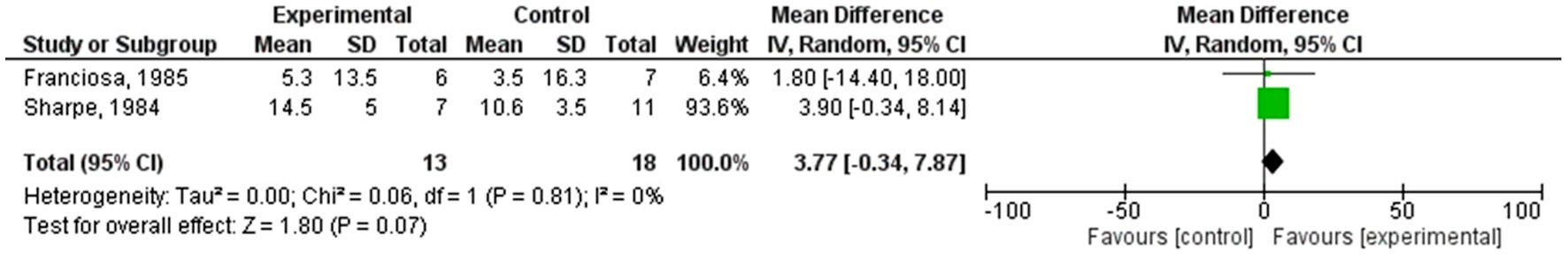 Click for large image | Figure 4. Forest plot of the improvement in left ventricular ejection fraction with ACE inhibitors. SD: standard deviation; CI: confidence interval; ACE: angiotensin-converting enzyme. |
| Discussion | ▴Top |
This systematic review and meta-analysis compiled data from 16 studies involving a total of 954 patients to evaluate the efficacy and safety of beta-blockers and ACE inhibitors in the treatment of chronic HF due to NIDCM. While we acknowledge that there have been tens of thousands of patients studied on beta-blockers and ACE inhibitors, our meta-analysis focuses specifically on NIDCM. This condition is a distinct subset of HF with unique pathophysiological characteristics and poorer prognosis. Therefore, our inclusion criteria were stringent to ensure that we analyzed studies that directly addressed the efficacy of these treatments in NIDCM patients. Although this resulted in a smaller sample size, it allowed us to provide a more precise and relevant analysis for this specific patient population.
The results of the meta-analysis showed that across 13 RCTs, beta-blocker therapy led to significant improvements in LVEF, exhibiting a large effect size (Cohen’s d = 1.30, 95% CI: 0.76 - 1.84) and statistical heterogeneity (I2 = 92%). The diversity observed among the beta-blocker studies highlights substantial variability in both patient responses and study methodologies. This variability, referred to as heterogeneity, indicates the extent of differences in the outcomes of various studies combined in the meta-analysis. In this case, the assessment of heterogeneity employed the I2 statistic and χ2 test [35, 36].
The notably high heterogeneity observed in the beta-blocker studies (I2 = 92%) indicates considerable divergence in treatment effects across these investigations. This variance likely stems from multifaceted factors: 1) Patient characteristics: Variability in treatment responses may be linked to differences in baseline attributes, including age, gender, comorbidities, and disease severity. Patients with NIDCM present diverse clinical profiles and underlying etiologies, which can influence their responsiveness to beta-blocker interventions. 2) Study designs: Variations in study structures, particularly in sample sizes, follow-up durations, and inclusion criteria, contribute significantly to this heterogeneity. The studies included in the meta-analysis exhibited varying participant counts, ranging from 14 to 383, and follow-up periods from 2 months to 2 years. Such discrepancies in study designs contribute to variability in treatment outcomes. 3) Treatment protocols: Heterogeneity may also arise from differences in the specific beta-blockers used, dosages, and treatment schedules across the studies. Diverse beta-blockers have distinct pharmacokinetic and pharmacodynamic profiles, which directly impact their efficacy in the NIDCM cohort. 4) Methodological variances: Differences in outcome measures, assessment methodologies, and statistical analyses employed in the studies further contribute to this heterogeneity. Variations in defining outcomes, such as changes in LVEF or QoL metrics, affect the interpretation of results.
The presence of significant heterogeneity in the effects of beta-blocker treatment for NIDCM necessitates caution when interpreting the collective effect size. This variability suggests potential differences in treatment effects across various patient populations and study settings. Consequently, careful consideration of individual study outcomes and their unique characteristics is crucial for prudent clinical decision-making.
Waagstein et al (1993) [19] reported improved clinical outcomes, including enhanced ejection fraction, better exercise performance, and increased hemodynamic stability due to treatment with metoprolol. These findings align with another study on chronic HF that observed similar benefits, including decreased mortality rates and reduced chances of adverse events, with prolonged beta-blocker use [37]. Seghatol et al (2004) [20] compared the efficacy of beta-blockers between ischemic and NIDCM. Due to differing pathophysiology, contractile reserve was identified as a valuable diagnostic and prognostic tool, predicting long-term outcomes in both scenarios. Combined with peripheral endothelial function, these findings facilitate mapping the potential results and duration of outcomes when using beta-blockers in NIDCM patients [38].
Numerous individual trials consistently demonstrate favorable outcomes associated with beta-blocker use in NIDCM patients. These benefits extend beyond improving LVEF, encompassing a range of enhancements in patients’ QoL. The improvement in QoL was measured using validated assessment tools such as the Minnesota Living with Heart Failure Questionnaire (MLHFQ) and the Kansas City Cardiomyopathy Questionnaire (KCCQ). Specific trials reported significant improvements in QoL scores, further supporting the comprehensive benefits of beta-blocker therapy in NIDCM patients. Patients treated with beta-blockers exhibit not only increased LVEF but also enhanced exercise tolerance and improved hemodynamic stability, as evidenced by improvements in end-diastolic and systolic pressures. Additionally, beta-blocker therapy correlates with reduced hospitalizations due to edema, worsening symptoms, and lower mortality rates among NIDCM patients [29-33].
One study highlighted cardiac remodeling as a key mechanism by which beta-blockers alleviate HF in NIDCM [27]. This concept is echoed in another study emphasizing that parameters of cardiac remodeling are important for prognosis and response to medication [39]. Other factors, such as race, age, genetics, New York Heart Association (NYHA) class, and baseline LVEF, have also been identified as indicative of prognosis [40]. In NIDCM patients, several factors independently predict adverse outcomes like death or the need for heart transplantation. These include a systolic LVEF below 35%, an extended QTc interval over 440 ms, and abnormal kidney function indicated by a glomerular filtration rate (GFR) below 60 mL/min/1.73 m2, all of which are independent prognostic factors for these serious outcomes in NIDCM patients [41, 42].
One study correlated cardiac tissue histology with prognosis, demonstrating that the extent of fibrosis significantly impacts the remodeling ability of beta-blockers [24]. Fibrosis is another prognostic factor that can interfere with not only normal cardiac cells but also hinder the effects of beta-blockers [43]. These studies have enhanced our understanding of the underlying mechanisms of deterioration in patients with NIDCM.
Subgroup analysis revealed that carvedilol resulted in greater ejection fraction improvements than metoprolol in treatment non-responders [23]. In our analysis, we compared the effects of carvedilol and metoprolol across multiple studies involving a total of 954 patients. The specific number of patients in each group varied across studies, but we ensured to include only those with sufficient sample sizes for statistical analysis. The comparison showed that carvedilol had a superior effect on LVEF and patient outcomes. While there was some variance between the studies, the overall effect size for carvedilol was significantly higher. This aligns with another study’s findings, which suggest carvedilol enhances survival chances [44]. Furthermore, our analysis also considered cardiovascular and all-cause mortality as important outcomes. We found that carvedilol not only improves LVEF but also significantly reduces both cardiovascular and all-cause mortality in patients with NIDCM. These findings support the comprehensive benefits of carvedilol in improving overall survival and reducing mortality risks, making it a robust choice for this patient population. Such outcomes may be attributed to the distinct pharmacological profile of third-generation beta-blockers. Carvedilol, unlike second-generation agents like metoprolol, demonstrates relative non-selectivity in blocking β1- and β2-receptors and additionally antagonizes α1-receptors, contributing to its vasodilatory properties [45]. Furthermore, carvedilol’s interaction with β-receptors involves an unusual engagement with G proteins, leading to receptor downregulation in experimental models, a response not seen with second-generation compounds. Carvedilol also exhibits potential antioxidant and antiproliferative properties, distinguishing it from its predecessors [46]. While both second- and third-generation β-blockers block β1-adrenergic receptors, the array of differences in their pharmacological characteristics suggests potential variations in their clinical effects, particularly in chronic HF.
In comparison, ACE inhibitors showed a beneficial role but with less pronounced effects. The meta-analysis of ACE inhibitors indicated a nonsignificant overall effect on LVEF improvement (Z = 1.36, P = 0.17; 95% CI: -0.24, 1.31) with low heterogeneity (Tau2 = 0.02; Chi2 = 1.09, df = 1, P = 0.30; I2 = 8%). The small number of studies (only two RCTs) and the nonsignificant results highlight the need for further research to determine the specific efficacy of ACE inhibitors in NIDCM.
The limitations of the ACE inhibitor data include the small number of studies and the high potential for bias due to the limited sample sizes. Additionally, the heterogeneity observed in the studies of beta-blockers (I2 = 92%) suggests substantial variability in treatment effects, potentially due to differences in patient characteristics, study designs, and treatment protocols. These factors must be considered when interpreting the results and their implications for clinical practice.
The proposed mechanisms of action for beta-blockers and ACE inhibitors involve attenuating the maladaptive neurohormonal cascade and preventing adverse cardiac remodeling, which are crucial to the pathogenesis of NIDCM [26]. Compared to previous HF meta-analyses, the improvement in LVEF appears more significant in this NIDCM-specific population, suggesting that tailored beta-blockade strategies may offer optimized outcomes. However, most of the trials included in the analysis were limited by small sample sizes and short follow-up durations, which may impact the generalizability of the findings.
Regarding ACE inhibitors, only two RCTs were examined, revealing nonsignificant pooled improvements in LVEF (MD: 0.11, 95% CI: -0.98 to 1.20) with minimal heterogeneity (I2 = 8%). While ACE inhibitors are commonly used in general HF patients, the specific efficacy of these drugs for NIDCM remains inconclusive due to the limited data available from these trials. We acknowledge that only two studies were used to evaluate ACE inhibitors, making the comparison weak. This limitation reflects the scarcity of research specifically addressing ACE inhibitors in NIDCM. Despite the limited number of studies, the analysis of these studies provides valuable preliminary insights. However, we emphasize the need for further research and larger-scale studies to robustly establish the efficacy and safety of ACE inhibitors in this specific patient population.
Cardiac remodeling is a process influenced by both beta-blockers and ACE inhibitors [47], and this commonality provides a rationale for their combined use in therapy to improve heart function [48]. Such conjunct therapy, leveraging the benefits of both medication classes, could potentially enhance treatment outcomes for patients with NIDCM. However, the need for more comprehensive and larger-scale studies is evident to confirm these findings and refine treatment guidelines for NIDCM.
ACE inhibitors are known to enhance stroke and cardiac indices while stabilizing pulmonary wedge pressures [49]. Although this has not been specifically studied in relation to NIDCM, it is understood to decrease the heart’s workload, thereby reducing both morbidity and mortality in patients [50, 51]. This aligns with a meta-analysis conducted to evaluate this particular aspect in patients with chronic HF [51]. Another favorable aspect of ACE inhibitors is their high tolerability among patients [51].
Additional large-scale RCTs with extended follow-up are necessary to clarify outstanding therapeutic questions. Nonetheless, currently available data indicate that beta-blockers can improve left ventricular performance and prognosis in NIDCM [20]. Results support updated practice recommendations advocating evidence-based beta-blockade for these patients [40, 41].
The conducted meta-analysis effectively addressed the research inquiries outlined in the introduction, aligning the findings with the hypothesized assertions. Primarily, the investigation aimed to evaluate the effectiveness of beta-blockers and ACE inhibitors in managing NIDCM. The analysis revealed a notable enhancement in LVEF associated with beta-blocker administration compared to control interventions, substantiating the initial hypothesis of significant improvement in cardiac function in NIDCM patients through beta-blocker therapy.
Conversely, the role of ACE inhibitors in NIDCM treatment remains inconclusive due to limited data. The insufficient evidence restricted the generalizability of conclusions regarding the efficacy of ACE inhibitors in this patient cohort, aligning with the initial hypothesis that highlighted the inconclusiveness of ACE inhibitor efficacy in NIDCM.
Secondary endpoints, including mortality rates, hospitalization frequency, and composite major adverse cardiac events, were examined. The outcomes suggested potential benefits associated with beta-blocker usage, notably in reducing mortality rates and hospital admissions among individuals with NIDCM. These findings are consistent with existing guidelines for ischemic cardiomyopathy, where beta-blockers exhibit a 34% decrease in risk, and ACE inhibitors show a 17% reduction [51]. However, these guidelines do not fully address the pathophysiology of various causes of NIDCM and the utilization and prioritization of one drug class over another. This meta-analysis emphasizes the importance of studying different cardiac indices before adopting and adhering to a specific treatment regimen.
Overall, this analysis confirms beta-blockers and ACE inhibitors as integral components of guideline-directed medical management for NIDCM-related chronic HF. The findings provide valuable insights that could potentially alter clinical practice in managing NIDCM. The breadth of data examined in this article spans a considerable timeframe, encompassing a vast reservoir of information meticulously scrutinized. Through this exhaustive analysis, the study unveils a treasure trove of invaluable insights that promise to not only steer future investigations but also fortify the foundation of knowledge underpinning the treatment of NIDCM across diverse clinical guidelines. By distilling this wealth of information into actionable results, the study serves as a beacon, illuminating pathways for enhanced therapeutic approaches and refined clinical strategies in the management of NIDCM. Specifically, the results suggest that beta-blockers should be considered the standard pharmacotherapy for NIDCM patients, while the role of ACE inhibitors is less definitively characterized.
Based on these findings, specific scenarios exist where beta-blockers might be more favorable than ACE inhibitors in the treatment of NIDCM: 1) Bleak-appearing case scenarios: The document underscores the role of carvedilol, a beta-blocker, in improving outcomes in bleak-appearing case scenarios [32]. In such cases, where patients have a relatively poorer prognosis, beta-blockers like carvedilol could be preferred over ACE inhibitors to positively impact prognosis and improve left ventricular performance. 2) Distinct pathophysiology: NIDCM exhibits distinct pathophysiological phenotypes, including higher levels of inflammatory biomarkers and increased cardiomyocyte apoptosis. The document suggests that the effectiveness of standard agents, such as ACE inhibitors, in other cohorts may not translate to NIDCM [20]. In these instances, beta-blockers may be more beneficial, as they have demonstrated efficacy in improving left ventricular performance and prognosis specifically in NIDCM.
It is important to note that while beta-blockers are recommended as the standard pharmacotherapy for NIDCM, ACE inhibitors still play a critical role in reducing adverse events and hospitalizations [34]. Therefore, a tailored approach that recognizes NIDCM’s distinct pathophysiology is crucial for optimizing treatment outcomes. Further research and adequately powered RCTs are needed to clarify the specific scenarios, in which beta-blockers may be more favorable than ACE inhibitors in NIDCM treatment and to develop evidence-based guidelines for tailored management strategies.
Limitations
In addition to the findings and conclusions previously discussed, it is crucial to acknowledge the limitations of the current meta-analysis. These limitations underscore areas where further research is needed to enhance our understanding and management of NIDCM.
Firstly, one limitation is the absence of standardized testing for improved QoL assurance. QoL is an essential aspect of patient care, and standardized measures are necessary to assess and monitor the impact of treatment interventions on patients’ well-being. Without such standardized testing, it becomes difficult to evaluate the true effectiveness of interventions, including beta-blockers and ACE inhibitors, in improving the QoL for NIDCM patients.
Secondly, there is a notable scarcity of study data available online that specifically and in-depth discusses ACE inhibitors in the context of NIDCM. This lack of comprehensive and targeted information limits our understanding of the role and efficacy of ACE inhibitors in managing NIDCM. For informed decision-making regarding the use of ACE inhibitors in this context, a robust body of evidence that directly addresses their effectiveness, safety, and optimal dosing regimens is essential.
Additionally, the current knowledge about NIDCM and its management is limited across various age groups. This suggests potential differences in pathophysiology, prognosis, and treatment response among different age cohorts with NIDCM. To provide personalized and effective care for NIDCM patients of all ages, research that specifically focuses on the distinct characteristics and needs of different age groups is imperative.
Furthermore, the RCTs included in this meta-analysis are somewhat dated, highlighting the need for more recent data to form better-informed opinions about current treatment regimens for NIDCM. Medical knowledge and treatment guidelines evolve, and it is crucial to stay current with the latest evidence to ensure optimal patient care. Conducting new RCTs with more recent data will provide valuable insights into the efficacy and safety of interventions, particularly beta-blockers and ACE inhibitors, in managing NIDCM.
The absence of up-to-date RCTs challenges our full understanding of the effectiveness and safety of specific interventions, such as ACE inhibitors, in the context of NIDCM. This limitation restricts the ability to assess the contemporary landscape of treatment options for NIDCM accurately. Medical management guidelines typically rely on the most current and robust evidence available; without recent RCTs, it becomes difficult to determine the optimal use of ACE inhibitors in patients with NIDCM, thus hindering healthcare professionals’ ability to make informed treatment decisions.
Moreover, the limited data on ACE inhibitors specifically targeted at NIDCM are a significant challenge. NIDCM is a distinct subset of cardiomyopathy with its own unique pathophysiology, necessitating research that specifically addresses the use of ACE inhibitors in this context. The absence of such data makes it difficult to ascertain the potential benefits and risks associated with the use of ACE inhibitors in patients with NIDCM.
The high heterogeneity observed in beta-blocker studies (I2 = 92%) suggests substantial differences in treatment effects across the studies, potentially due to varying study designs, treatment protocols, and other factors. Conducting RCTs with standardized protocols could provide a more consistent understanding of the role of beta-blockers in NIDCM, enhancing the reliability of conclusions drawn from such studies.
Conclusions
This first systematic evaluation provides evidence supporting the efficacy of beta-blockers in NIDCM, while the role of ACE inhibitors is less definitively characterized and requires studies specifically tailored to NIDCM. Beta-blockers showed a significant improvement in LVEF with an effect size of Cohen’s d = 1.30 (95% CI: 0.76, 1.84; Z = 4.72, P < 0.00001), despite high heterogeneity (I2 = 92%). In contrast, ACE inhibitors demonstrated a nonsignificant overall effect on LVEF improvement (Z = 1.36, P = 0.17; 95% CI: -0.24, 1.31) with low heterogeneity (I2 = 8%). Based on our analysis, we recommend carvedilol for its superior efficacy in improving LVEF and patient outcomes in NIDCM, with a number needed to treat (NNT) of approximately 8 for significant improvement. This NNT reflects the robust efficacy of carvedilol in this specific patient population.
These findings highlight the clinical relevance of beta-blockers, particularly carvedilol, which should be considered as first-line therapy in managing NIDCM due to their significant impact on improving LVEF. The study underscores the need for further research on ACE inhibitors to better determine their specific efficacy in this patient population. Nonetheless, current data indicate that evidence-based neurohormonal antagonism with beta-blockers can favorably impact the prognosis in NIDCM, and ACE inhibitors are critical in decreasing adverse events and hospitalizations in patients’ lives.
The poor prognosis and lack of clear optimal management highlight the importance of the information in this review. Understanding and comparing the differences between treatments that have been studied and tested for NIDCM is crucial for guiding clinical decisions and improving patient outcomes in this high-risk population. Implementing these insights can enhance treatment strategies and improve patient outcomes in NIDCM.
Learning points
Beta-blockers, notably carvedilol, significantly improve LVEF and prognosis in NIDCM, outperforming metoprolol, particularly in treatment-resistant cases.
Carvedilol shows greater efficacy than metoprolol in NIDCM, enhancing LVEF and patient outcomes, especially in complex clinical scenarios.
Findings advocate a tailored NIDCM treatment, prioritizing beta-blockers like carvedilol, while recommending a cautious approach to ACE inhibitors until further evidence is available.
The role of ACE inhibitors in NIDCM is unclear, highlighting the need for targeted research and more RCTs to determine their specific effectiveness.
Urgent, updated research is needed on NIDCM, focusing on ACE inhibitors, to optimize treatment strategies for this unique condition.
| Supplementary Material | ▴Top |
Suppl 1. Comprehensive search strategy.
Acknowledgments
The authors have no acknowledgements to declare, reflecting the independent completion of the work.
Financial Disclosure
No funding was received for the conduct of this study or the preparation of this article, indicating that there are no financial sources to declare.
Conflict of Interest
The authors declare no conflicts of interest to ensure the impartiality of the review.
Informed Consent
Not applicable. This research is a systematic review and meta-analysis based on previously published data; therefore, informed consent from participants is not required.
Author Contributions
Jordan Llerena-Velastegui, MD: supervision, conceptualization, writing - original draft, writing - review and editing. Melisa Santamaria-Lasso, MD: supervision, writing - review and editing. Melany Mejia-Mora, MD: data curation, writing - review and editing. Mauricio Santander-Aldean, MD: formal analysis, writing - review and editing. Andrea Granda-Munoz, MD: formal analysis, writing - original draft, supervision. Claudia Hurtado-Alzate, MD: data curation, formal analysis, writing - original draft. Ana Clara Fonseca Souza de Jesus, MD: data curation, formal analysis, writing - original draft. Jurgen Baldelomar-Ortiz, MD: data curation, writing - review and editing, methodology.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- McKenna WJ, Maron BJ, Thiene G. Classification, epidemiology, and global burden of cardiomyopathies. Circ Res. 2017;121(7):722-730.
doi pubmed - Cecchi F, Tomberli B, Olivotto I. Clinical and molecular classification of cardiomyopathies. Glob Cardiol Sci Pract. 2012;2012(1):4.
doi pubmed pmc - Fuster V, Gersh BJ, Giuliani ER, Tajik AJ, Brandenburg RO, Frye RL. The natural history of idiopathic dilated cardiomyopathy. Am J Cardiol. 1981;47(3):525-531.
doi pubmed - Keeling PJ, Goldman JH, Slade AK, Elliott PM, Caforio AL, Poloniecki J, McKenna WJ. Prognosis of idiopathic dilated cardiomyopathy. J Card Fail. 1995;1(5):337-345.
doi pubmed - Hellawell JL, Margulies KB. Myocardial reverse remodeling. Cardiovasc Ther. 2012;30(3):172-181.
doi pubmed - Lopes Fernandes S, Ribeiro Carvalho R, Graça Santos L, Montenegro Sá F, Ruivo C, Lázaro Mendes S, Martins H, et al. Fisiopatologia e Tratamento da Insuficiencia Cardiaca com Fracao de Ejecao Preservada: Estado da Arte e Perspectivas para o Futuro. Arquivos Brasileiros De Cardiologia [Internet]. 2020;141(1):120-129.
doi - Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54(19):1747-1762.
doi pubmed - Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116(7):1254-1268.
doi pubmed pmc - Morita H, Seidman J, Seidman CE. Genetic causes of human heart failure. J Clin Invest. 2005;115(3):518-526.
doi pubmed pmc - Groenning BA, Nilsson JC, Sondergaard L, Fritz-Hansen T, Larsson HB, Hildebrandt PR. Antiremodeling effects on the left ventricle during beta-blockade with metoprolol in the treatment of chronic heart failure. J Am Coll Cardiol. 2000;36(7):2072-2080.
doi pubmed - Gilbert EM, Abraham WT, Olsen S, Hattler B, White M, Mealy P, Larrabee P, et al. Comparative hemodynamic, left ventricular functional, and antiadrenergic effects of chronic treatment with metoprolol versus carvedilol in the failing heart. Circulation. 1996;94(11):2817-2825.
doi pubmed - Patten RD, Konstam MA. Ventricular remodeling and the renin angiotensin aldosterone system. Congest Heart Fail. 2000;6(4):187-192.
doi pubmed - Struthers AD. Aldosterone escape during angiotensin-converting enzyme inhibitor therapy in chronic heart failure. J Card Fail. 1996;2(1):47-54.
doi pubmed - Linz W, Wiemer G, Scholkens BA. Beneficial effects of bradykinin on myocardial energy metabolism and infarct size. Am J Cardiol. 1997;80(3A):118A-123A.
doi pubmed - Petrich BG, Eloff BC, Lerner DL, Kovacs A, Saffitz JE, Rosenbaum DS, Wang Y. Targeted activation of c-Jun N-terminal kinase in vivo induces restrictive cardiomyopathy and conduction defects. J Biol Chem. 2004;279(15):15330-15338.
doi pubmed - Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst Rev. 2022;18(2):e1230.
doi pubmed pmc - Chalmers TC, Frank CS, Reitman D. Minimizing the three stages of publication bias. JAMA. 1990;263(10):1392-1395.
pubmed - Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
doi pubmed pmc - Waagstein F, Bristow MR, Swedberg K, Camerini F, Fowler MB, Silver MA, Gilbert EM, et al. Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Metoprolol in Dilated Cardiomyopathy (MDC) Trial Study Group. Lancet. 1993;342(8885):1441-1446.
doi pubmed - Seghatol FF, Shah DJ, Diluzio S, Bello D, Johnson MR, Cotts WG, O'Donohue JA, et al. Relation between contractile reserve and improvement in left ventricular function with beta-blocker therapy in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2004;93(7):854-859.
doi pubmed - Patrianakos AP, Parthenakis FI, Mavrakis HE, Saatsaki M, Diakakis GF, Chlouverakis GI, Vardas PE. Effects of Nebivolol on left ventricular function and exercise capacity in patients with non-ischaemic dilated cardiomyopathy. A randomised placebo-controlled study. Hellenic J Cardiol. 2005;46(3):199-207.
pubmed - Lowes BD, Gilbert EM, Abraham WT, Minobe WA, Larrabee P, Ferguson D, Wolfel EE, et al. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med. 2002;346(18):1357-1365.
doi pubmed - Yamada T, Fukunami M, Ohmori M, Iwakura K, Kumagai K, Kondoh N, Minamino T, et al. Which subgroup of patients with dilated cardiomyopathy would benefit from long-term beta-blocker therapy? A histologic viewpoint. J Am Coll Cardiol. 1993;21(3):628-633.
doi pubmed - Ng AC, Sindone AP, Wong HS, Freedman SB. Differences in management and outcome of ischemic and non-ischemic cardiomyopathy. Int J Cardiol. 2008;129(2):198-204.
doi pubmed - Parent JJ, Towbin JA, Jefferies JL. Medical Therapy Leads to Favorable Remodeling in Left Ventricular Non-compaction Cardiomyopathy: Dilated Phenotype. Pediatr Cardiol. 2016;37(4):674-677.
doi pubmed - Hall SA, Cigarroa CG, Marcoux L, Risser RC, Grayburn PA, Eichhorn EJ. Time course of improvement in left ventricular function, mass and geometry in patients with congestive heart failure treated with beta-adrenergic blockade. J Am Coll Cardiol. 1995;25(5):1154-1161.
doi pubmed - Olsen SL, Gilbert EM, Renlund DG, Taylor DO, Yanowitz FD, Bristow MR. Carvedilol improves left ventricular function and symptoms in chronic heart failure: a double-blind randomized study. J Am Coll Cardiol. 1995;25(6):1225-1231.
doi pubmed - Gilbert EM, Anderson JL, Deitchman D, Yanowitz FG, O'Connell JB, Renlund DG, Bartholomew M, et al. Long-term beta-blocker vasodilator therapy improves cardiac function in idiopathic dilated cardiomyopathy: a double-blind, randomized study of bucindolol versus placebo. Am J Med. 1990;88(3):223-229.
doi pubmed - Metra M, Nardi M, Giubbini R, Dei Cas L. Effects of short- and long-term carvedilol administration on rest and exercise hemodynamic variables, exercise capacity and clinical conditions in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1994;24(7):1678-1687.
doi pubmed - Di Lenarda A, Sabbadini G, Salvatore L, Sinagra G, Mestroni L, Pinamonti B, Gregori D, et al. Long-term effects of carvedilol in idiopathic dilated cardiomyopathy with persistent left ventricular dysfunction despite chronic metoprolol. The Heart-Muscle Disease Study Group. J Am Coll Cardiol. 1999;33(7):1926-1934.
doi pubmed - Sanderson JE, Chan SK, Yip G, Yeung LY, Chan KW, Raymond K, Woo KS. Beta-blockade in heart failure: a comparison of carvedilol with metoprolol. J Am Coll Cardiol. 1999;34(5):1522-1528.
doi pubmed - Quaife RA, Gilbert EM, Christian PE, Datz FL, Mealey PC, Volkman K, Olsen SL, et al. Effects of carvedilol on systolic and diastolic left ventricular performance in idiopathic dilated cardiomyopathy or ischemic cardiomyopathy. Am J Cardiol. 1996;78(7):779-784.
doi pubmed - Franciosa JA, Wilen MM, Jordan RA. Effects of enalapril, a new angiotensin-converting enzyme inhibitor, in a controlled trial in heart failure. J Am Coll Cardiol. 1985;5(1):101-107.
doi pubmed - Sharpe DN, Murphy J, Coxon R, Hannan SF. Enalapril in patients with chronic heart failure: a placebo-controlled, randomized, double-blind study. Circulation. 1984;70(2):271-278.
doi pubmed - Cleland JG, Dargie HJ, Ball SG, Gillen G, Hodsman GP, Morton JJ, East BW, et al. Effects of enalapril in heart failure: a double blind study of effects on exercise performance, renal function, hormones, and metabolic state. Br Heart J. 1985;54(3):305-312.
doi pubmed pmc - Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362(9377):7-13.
doi pubmed - Xie X, Wu B, Chen Y, Li W, Hu X, Chen J. Peripheral endothelial function may predict the effectiveness of beta-blocker therapy in patients with idiopathic dilated cardiomyopathy. Int J Cardiol. 2016;221:128-133.
doi pubmed - Japp AG, Gulati A, Cook SA, Cowie MR, Prasad SK. The diagnosis and evaluation of dilated cardiomyopathy. J Am Coll Cardiol. 2016;67(25):2996-3010.
doi pubmed - Kelesidis I, Hourani P, Varughese C, Zolty R. Effect of race on left ventricular ejection fraction decline after initial improvement with beta blockers in patients with non-ischemic cardiomyopathy: a retrospective analysis. Drugs R D. 2013;13(3):183-190.
doi pubmed pmc - Karatolios K, Holzendorf V, Richter A, Schieffer B, Pankuweit S, Competence Network Heart Failure G. Long-term outcome and predictors of outcome in patients with non-ischemic dilated cardiomyopathy. Int J Cardiol. 2016;220:608-612.
doi pubmed - Cojan-Minzat BO, Zlibut A, Agoston-Coldea L. Non-ischemic dilated cardiomyopathy and cardiac fibrosis. Heart Fail Rev. 2021;26(5):1081-1101.
doi pubmed - Ruffolo RR, Jr., Gellai M, Hieble JP, Willette RN, Nichols AJ. The pharmacology of carvedilol. Eur J Clin Pharmacol. 1990;38(Suppl 2):S82-88.
doi pubmed - Bristow MR, Larrabee P, Muller-Beckmann B, Minobe W, Roden R, Skerl L, Klein J, et al. Effects of carvedilol on adrenergic receptor pharmacology in human ventricular myocardium and lymphocytes. Clin Investig. 1992;70(Suppl 1):S105-113.
doi pubmed - Sigurdsson A, Swedberg K. Neurohormonal activation and congestive heart failure: today's experience with ACE inhibitors and rationale for their use. Eur Heart J. 1995;16(Suppl N):65-72.
doi pubmed - Syed T, Lal B, Ahir D, et al. Effective high dose Multi-Drug treatment regimen favours event free survival rate in DCMP patients. ResearchGate [Internet]. 2021. Available from: https://www.researchgate.net/publication/350240922_Effective_High_Dose_Multi-Drug_Treatment_Regimen_Favours_Event_Free_Survival_Rate_in_DCMP_Patients?enrichId=rgreq-14bdc4139ef00a6296416c3cf25ebb0e-.
- Cody RJ, Covit AB, Schaer GL, Laragh JH. Evaluation of a long-acting converting enzyme inhibitor (enalapril) for the treatment of chronic congestive heart failure. J Am Coll Cardiol. 1983;1(4):1154-1159.
doi pubmed - Solvd Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293-302.
doi pubmed - Creager MA, Massie BM, Faxon DP, Friedman SD, Kramer BL, Weiner DA, Ryan TJ, et al. Acute and long-term effects of enalapril on the cardiovascular response to exercise and exercise tolerance in patients with congestive heart failure. J Am Coll Cardiol. 1985;6(1):163-173.
doi pubmed - Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA. 1995;273(18):1450-1456.
pubmed - Kjekshus J, Swedberg K. Tolerability of enalapril in congestive heart failure. Am J Cardiol. 1988;62(2):67A-72A.
doi pubmed - Chopra V, Kakarla S, Oomman A. Management of non-ischemic dilated cardiomyopathy. Heart Failure Journal of India [Internet]. 2023;1(1):31.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


