| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 15, Number 3, June 2024, pages 134-143
Association Between Major Adverse Cardiovascular Events and Left Ventricular Mass Index in Patients Who Have Undergone Coronary Computed Tomography Angiography: From the FU-CCTA Registry
Tetsuro Tachibanaa, Yuhei Shigaa, Kohei Tashiroa, Sara Higashia, Yuka Shibataa, Yuto Kawahiraa, Yuta Katoa, Takashi Kuwanoa, Makoto Sugiharaa, Shin-ichiro Miuraa, b, c
aDepartment of Cardiology, Fukuoka University School of Medicine, Fukuoka, Japan
bDepartment of Cardiology, Fukuoka University Nishijin Hospital, Fukuoka, Japan
cCorresponding Author: Shin-ichiro Miura, Department of Cardiology, Fukuoka University School of Medicine, Fukuoka 814-0180, Japan
Manuscript submitted April 30, 2024, accepted June 7, 2024, published online June 25, 2024
Short title: MACEs and LVMI
doi: https://doi.org/10.14740/cr1655
| Abstract | ▴Top |
Background: Left ventricular mass (LVM) is a predictor of future cardiovascular risk. We determined the association between LVM measured by coronary computed tomography angiography (CCTA) and the prognosis in patients who have undergone CCTA for screening of coronary artery disease (CAD).
Methods: We performed a prospective cohort study. Five hundred twenty consecutive patients who underwent CCTA at Fukuoka University Hospital (FU-CCTA registry) were enrolled. They were clinically suspected of having CAD or had at least one cardiovascular risk factor, and were a follow-up of up to 5 years. Equal to more than 50% of coronary stenosis as assessed by CCTA was diagnosed as CAD. Using CCTA, LVM index (LVMI), LV ejection fraction (LVEF), LV end-diastolic volume (LVEDV) and LV end-systolic volume were measured. The primary endpoint was major adverse cardiovascular events (MACEs: including all causes of death, ischemic stroke, acute myocardial infarction and coronary revascularization). The patients were divided into non-MACEs and MACEs groups.
Results: The non-MACEs and MACEs groups consisted of 478 and 42 patients, respectively. Percent of CAD in the MACEs group was significantly higher than that in the non-MACEs group. The MACEs group showed significantly higher LVMI and tended to have a lower LVEF and LVEDV than the non-MACEs group. Although LVMI was not associated with MACEs in all patients, LVMI was independently associated with MACEs in males (odd ratio: 1.018, 95% confidence interval: 1.002 - 1.035, P = 0.030), but not females.
Conclusions: Evaluation of LVMI by CCTA may be useful for predicting MACEs in males.
Keywords: Major adverse cardiovascular events; Left ventricular mass; Coronary computed tomography angiography
| Introduction | ▴Top |
We have been studying risk factors for coronary artery disease (CAD) in a registry using coronary computed tomography angiography (CCTA) at Fukuoka University Hospital (FU-CCTA registry) for the primary prevention of CAD [1-11]. CCTA is a suitable strategy for screening of CAD, with a sensitivity of 89% and specificity of 96%, a positive predictive value of 78%, and a negative predictive value of 98% [12, 13]. CCTA is also becoming more widely available in many general hospitals, allowing the noninvasive evaluation of coronary artery stenosis, calcification, and plaque [14, 15]. CCTA can also be used to measure left ventricular ejection fraction (LVEF) and LV mass (LVM) with the use of software [16-18].
LV hypertrophy (LVH) is a predictor of cardiovascular morbidity and mortality [19-22], and the improvement of LVH reduces the subsequent cardiovascular risk [23, 24]. The Framingham study showed that an increase in LVM of 50 g per height, as measured by echocardiography, was associated with an increased risk of CAD in both males and females [20, 22]. Thus, an increased LVM has been associated with CAD [25, 26]. Although LV volume is routinely calculated from end-diastolic cross-sectional images using echocardiography, echocardiography is not always accurate due to problems such as poor delineation caused by obesity, lungs, and the skill of the examiner [27, 28]. Reproducibility of main echocardiographic indices of left ventricular systolic function is strongly influenced by the chest wall conformation [29].
Recent studies have also shown that LVM measured by CCTA correlates with coronary atherosclerosis at the time of the examination and is an independent risk factor for myocardial ischemia [25, 30]. Although an increased LVM was suggested to be a possible prognostic factor for major adverse cardiovascular events (MACEs) and all-cause mortality [30], it was not shown to be confounded by other cardiovascular risks. Therefore, we hypothesized that LV myocardial weight on CCTA examination could be a good prognostic predictor of MACEs. Using the FU-CCTA registry of patients undergoing CCTA at Fukuoka University, we investigated the association between LVM on CCTA and subsequent MACEs.
| Materials and Methods | ▴Top |
Study subjects
A prospective cohort study was conducted. We enrolled 520 consecutive patients who underwent CCTA for screening of CAD at Fukuoka University Hospital (FU-CCTA registry) and either were clinically suspected of having CAD or had at least one cardiovascular risk factor. They were follow-up of up to 5 years (3.5 ± 0.64 years). The study was approved by the Fukuoka University Ethics Committee (#09-10-02) and was registered at the UMIN Clinical Trials Registry (#000016641, 25/02/2015). All the participants were provided with the informed consent form before enrollment and all the protocol was performed according to the Declaration of Helsinki (Fortaleza, October 13, 2013). Patients with Cr > 2.0 mg/dL or contrast agent allergy were excluded in this study.
Coronary artery evaluation
Coronary artery stenosis was evaluated by constructing a volume-rendered image using Aquilion ONE, a slice CT manufactured by Cannon, and evaluating the degree of lumen stenosis with multiplanar images. Using CCTA, ≥ 50% of coronary stenosis was considered to be CAD according to the Society of Cardiovascular Computed Tomography Guidelines [31]. The severity of coronary artery stenosis was determined by the number of lesion branches, the coronary artery calcification (CAC) score, and the Gensini score [32]. The cardiac function analysis function installed in Aquilion ONE was used to automatically trace and analyze both diastolic and systolic cardiac function, and LVM, LVEF, left ventricular end-diastolic volume (LVEDV), and left ventricular end-systolic volume (LVESV) were measured. The LVM index (LVMI) was calculated by dividing the LVM by the body surface area (BSA) of each patient (Fig. 1). BSA was calculated by Du Bois formula (height (m)0.725 × weight (kg)0.425 × 0.007184) [33].
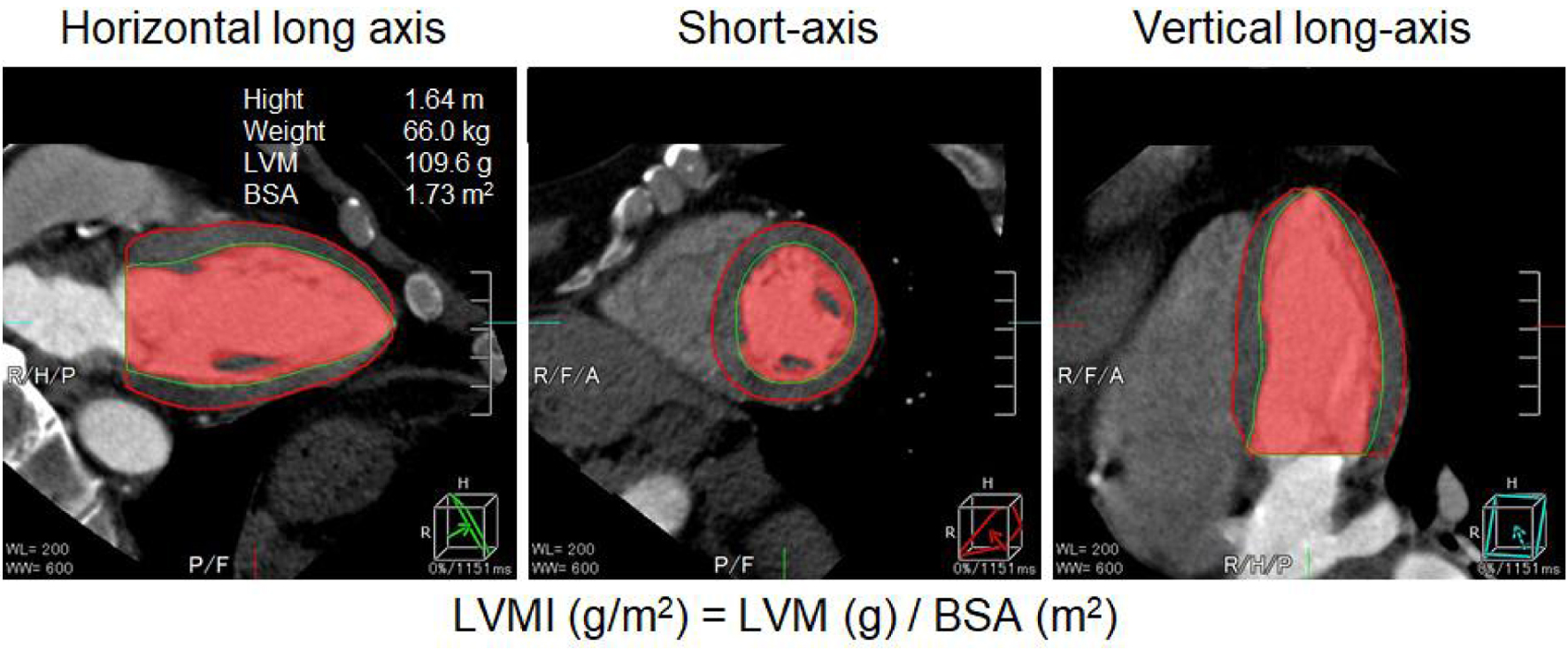 Click for large image | Figure 1. LVMI was calculated by dividing the LVM by the BSA of the patient. LVMI: left ventricular volume index; BSA: body surface area. |
Cardiovascular risk assessment
Cardiovascular risk factors in this study were defined as hypertension, family history of CAD, smoking history, dyslipidemia, diabetes, chronic kidney disease, and metabolic syndrome. All patients were evaluated with regard to body mass index (BMI), family history of CAD, smoking history, blood pressure, diabetes, hemoglobin A1c (HbA1c), and fasting blood glucose. In addition, the presence of dyslipidemia and triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), non-HDL-C, chronic kidney disease, and estimated glomerular filtration rate (eGFR) was assessed. Metabolic syndrome (MetS) was defined as at least two of a visceral fat area of 100 cm2 or more, TG of 150 mg/dL or more or HDL-C of 40 mg/dL or less, fasting blood glucose of 110 mg/dL or more, systolic blood pressure of 130 mm Hg or more and a diastolic blood pressure of 85 mm Hg or more [34].
Medication
Patients were being treated with an angiotensin-converting-enzyme inhibitor/angiotensin II receptor blocker (ACEi/ARB), β-blocker, calcium channel blocker (CCB), or diuretic (DU) for hypertension. They were taking statins or eicosapentaenoic acid (EPA) for dyslipidemia, and α-glucosidase inhibitor (α-GI), biguanide, dipeptidyl peptidase-4 inhibitor (DPP-4i), insulin, sulfonylurea (SU) or thiazolizine for diabetes.
Primary endpoints
The primary endpoints were defined as all causes of death, ischemic stroke, myocardial infarction, and coronary artery revascularization (MACEs), and patients were divided into two groups: MACEs and non-MACEs.
Statistical analysis
Statistical analyses were performed using Excel Statistics 2016 (SSRI, Tokyo, Japan) at Fukuoka University (Fukuoka, Japan) and the Stat View statistical software package (Stat View 5; SAS Institute Inc., Cary, NC, USA). Continuous variables are shown as the mean ± standard deviation (SD). Categorical and continuous variables were compared between groups using a Chi-squared analysis and t-tests, respectively. Univariate and multivariate logistic regression analysis was used to identify independent variables that were related to the presence or absence of MACEs. A value of P < 0.05 was considered significant.
| Results | ▴Top |
Patient characteristics at baseline for all patients in the MACEs and non-MACEs groups
Patient characteristics at baseline in all patients are shown in Table 1. The total population consisted of 261 (50.2%) males and 259 (49.8%) females, and the mean age was 66 ± 11 years. The MACEs and non-MACEs groups consisted of 42 and 478 patients, respectively. The percentage of males among patients with MACEs was significantly greater than the percentage of those in the non-MACEs group (73.8% vs. 48.1%, P < 0.05). The overall BMI was 23.9 ± 3.6 kg/m2, 22.9% had a family history of CAD, and 37.5% had a history of smoking, and this latter characteristic was significantly more common in the MACEs group (57.1% vs. 35.8%, P < 0.05). The mean systolic and diastolic blood pressures were 136 ± 20 and 77 ± 13 mm Hg, respectively, among patients with hypertension (68.8% of the total), and the mean HbA1c was 6.0±1.1% in those with diabetes mellitus (DM) (23.8%). Dyslipidemia was seen in 59.8% of the population, with mean TG 134 ± 94 mg/dL, HDL-C 55 ± 15 mg/dL, LDL-C 113 ± 32 mg/dL, and non-HDL-C 142 ± 39 mg/dL. Chronic kidney disease was seen in 29.8% of the population and the mean eGFR was 67.2 ± 16.2 mL/min/1.73 m2. The CAC and Gensini scores were 750 ± 1,545 and 26.5 ± 32.5 in the MACEs group and 218 ± 585 and 11.1 ± 12.8 in the non-MACEs group, respectively. There were no significant differences in ACEi, ARB, or other oral therapies between the two groups.
 Click to view | Table 1. All Patient Characteristics at Baseline in Non-MACEs and MACEs Groups |
LV profiles for all patients in the non-MACEs and MACEs groups
Next, LV function on CT was examined in the MACEs and non-MACEs groups (Fig. 2). While LVEF, LVEDV, and LVESV were not significantly different between the two groups, LVMI was significantly increased in the MACEs group (68.2 ± 22.5 vs. 77.2 ± 27.0 g/m2, P < 0.05).
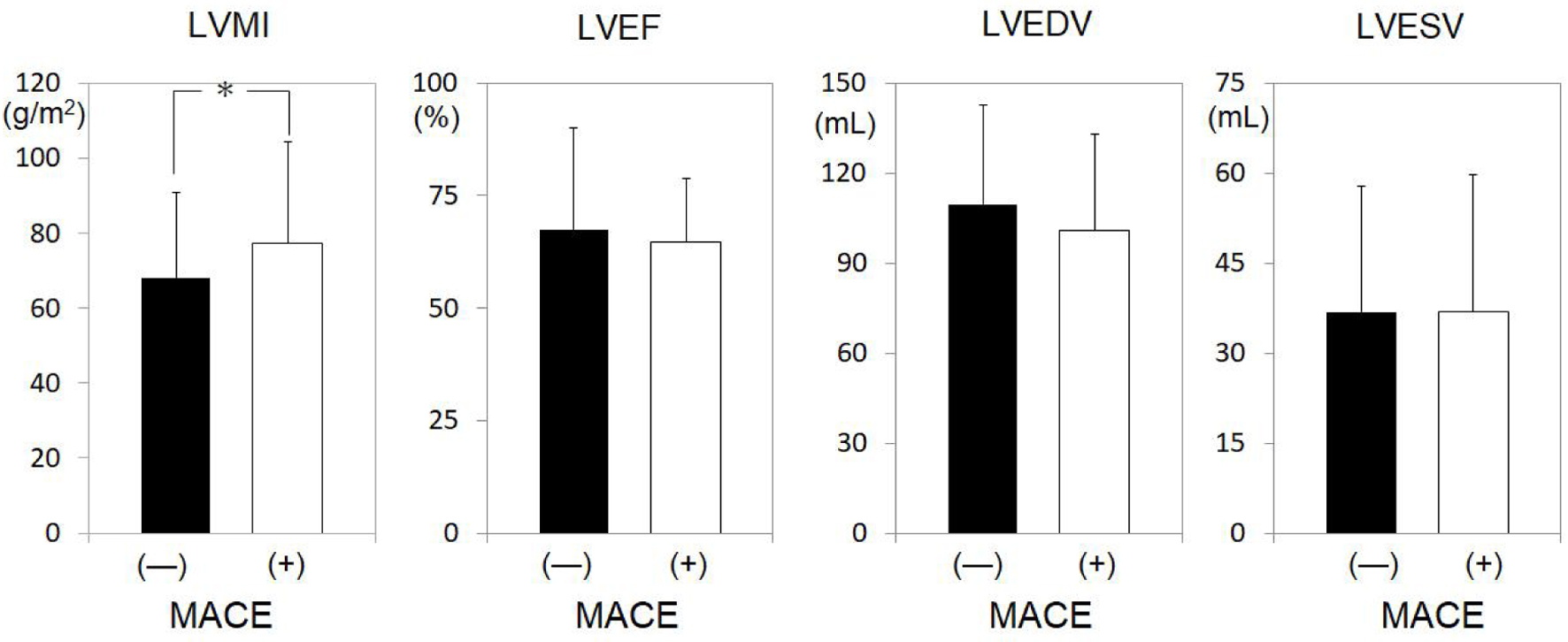 Click for large image | Figure 2. LV profiles in all patients in the non-MACEs and MACEs groups. *P < 0.05. LV: left ventricle; MACEs: major adverse cardiovascular events; LVMI: LV mass index; LVEF: LV ejection fraction; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume; N.S.: not significant. |
Patient characteristics at baseline for males and females in the non-MACEs and MACEs groups
The MACEs and non-MACEs groups were divided according to gender (Tables 2 and 3), since the MACEs group had a significantly greater proportion of males. Among just males (Table 2), significant differences between the non-MACEs and MACEs groups were observed for age (63 ± 12 vs. 68 ± 13 years, P = 0.028) and BMI (24.2 ± 3.4 vs. 22.9 ± 3.0 kg/m2, P = 0.042). Among just females (Table 3), significant differences between the non-MACEs and MACEs groups were found for smoking history (12.5% vs. 36.4%, P = 0.028) and non-HDL-C (147 ± 37 vs. 119 ± 63 mg/dL, P = 0.017). Next, LV function was examined for males and females in the MACEs and non-MACEs groups (Figs. 3 and 4). In males, LVEF (66.2±9.2% vs. 61.6±13.9%, P = 0.016) and LVEDV (118.4 ± 34.7 vs. 102.4 ± 35.3 mL, P = 0.042) were significantly lower in the MACEs group compared to the non-MACEs group (Fig. 3). However, there was no significant difference in cardiac function among females (Fig. 4).
 Click to view | Table 2. Patient Characteristics at Baseline in Non-MACEs and MACEs Groups in Males |
 Click to view | Table 3. Patient Characteristics at Baseline in Non-MACEs and MACEs Groups in Females |
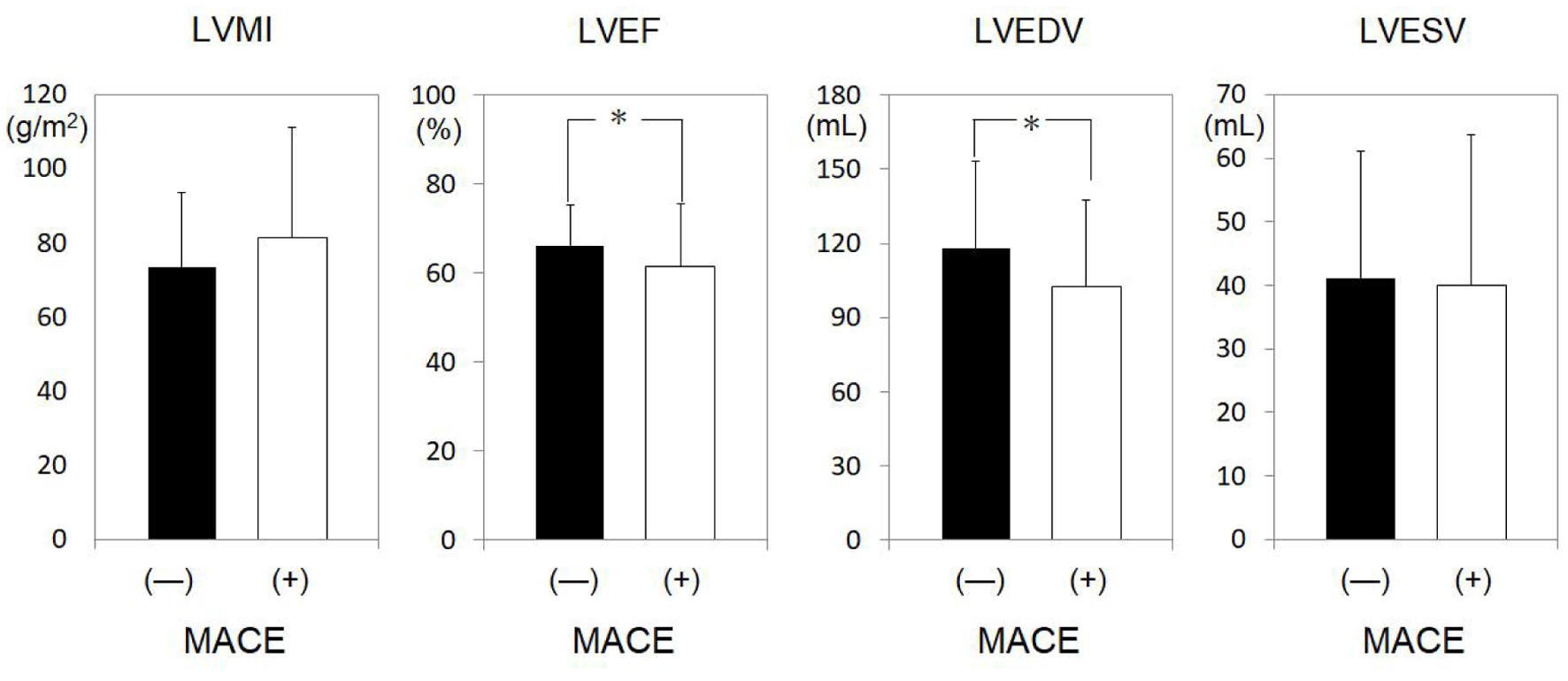 Click for large image | Figure 3. LV profiles in males in the non-MACEs and MACEs groups. *P < 0.05. LV: left ventricle; MACEs: major adverse cardiovascular events; LVMI: LV mass index; LVEF: LV ejection fraction; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume; N.S.: not significant. |
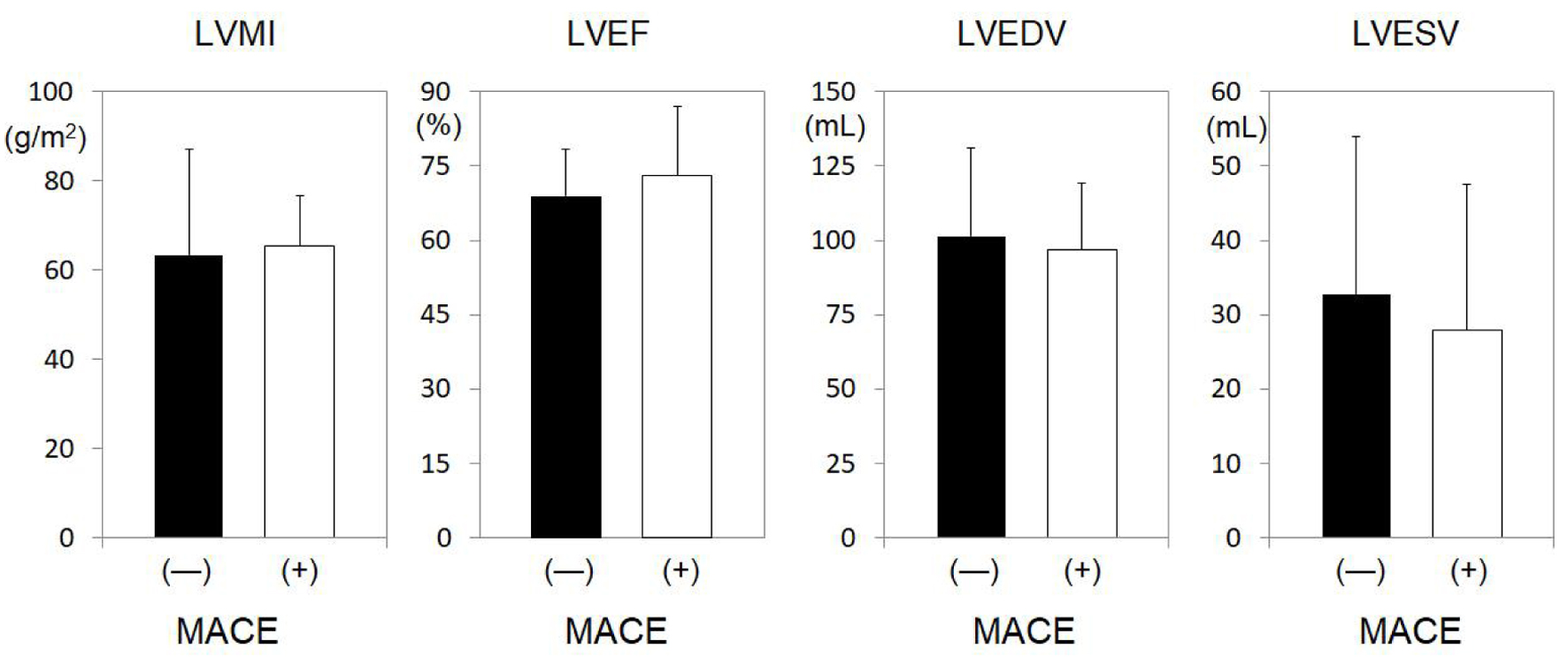 Click for large image | Figure 4. LV profiles in females in the non-MACEs and MACEs groups. *P < 0.05. LV: left ventricle; MACEs: major adverse cardiovascular events; LVMI: LV mass index; LVEF: LV ejection fraction; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume; N.S.: not significant. |
Predictors for the presence of MACEs in all patients, in the males and females
Univariate logistic regression analysis of risk factors for MACEs in all patients showed that males (P = 0.002), smoking (P = 0.007) and LVMI (P = 0.024) were significantly associated with MACEs (Table 4). We next performed this analysis separately for males and females (Table 4). In males, age (P = 0.029) and BMI (P = 0.042) were associated with MACEs, whereas LVMI was not associated. In contrast, in females, only smoking (P = 0.035) was associated with MACEs.
 Click to view | Table 4. The Univariate and Multivariate Logistic Regression Analysis of Risk Factors for MACEs |
Next, multivariate logistic regression analysis was performed to correct for each confounding factor. Risk factors for MACEs showed that being male was significantly associated with MACEs, with an odds ratio (OR) of 2.457 (P = 0.039), while LVMI was not significantly associated with MACEs, with an OR of 1.011 (P = 0.072). Next, this analysis was conducted separately for males and females (Table 4). Among just males, LVMI was significantly different between the MACEs and non-MACEs, with an OR of 1.018 (P = 0.030) (Table 4). On the other hand, among only females, the MACEs and non-MACEs groups significantly differed with respect to smoking history, with an OR of 4.68 (P = 0.029), but not LVMI, with an odds ratio of 1.062 (P = 0.867) (Table 4).
| Discussion | ▴Top |
The main finding in this study was that, among all patients, LVMI was significantly higher in the MACEs group than in the non-MACEs group. On the other hand, there were no significant differences in LVEF, LVEDV, or ESV between the two groups. In the logistic regression analysis in the total population, only the male gender, but not LVMI, was associated with MACEs. Finally, MACEs were shown to be associated with LVMI in males, but not females.
Although electrocardiography is widely used to evaluate LVH, its high specificity but low sensitivity can make accurate assessment challenging [35]. Echocardiography studies, but not electrocardiography studies, have suggested that a 1 g/m2 increase in LVMI is associated with a 3% increase in the hazard ratio for heart failure [36], and an increased LVMI is associated with all-cause mortality, arrhythmic death and sudden death in patients with CAD [37]. Furthermore, from the Framingham study, an increase in LVM by echocardiography over time in a healthy population is known to be a risk factor for MACEs [20, 22]. Each increase in LVMI by coronary angiography and subsequent myocardial assessment over time using cardiac magnetic resonance imaging increases the risk of all-cause mortality and the need for coronary revascularization therapy [38]. It has also been shown that LVMI at the time of measurement using CCTA is associated with MACEs and all-cause mortality [30]. Unfortunately, both studies evaluated secondary prevention, but not primary prevention, in populations of patients with pre-existing CAD [30, 38]. The risk of MACEs in the target group was relatively high because the patients required secondary prevention [30]. Furthermore, since the study model involved gender-matched MACEs and non-MACEs groups, the differences between males and females could not be determined. Previously, it was not clear whether LVMI is associated with primary prevention of MACEs together with gender differences, as in the present study. However, in our study, LVMI was associated with the primary prevention of MACEs with regard to a gender difference.
In this study, we evaluated the correlation between LVMI and MACEs at the time of measurement and were able to show that LVMI was significantly higher in the MACEs group than in the non-MACEs group, confirming the trend observed in previous studies [30, 38]. Our study showed that being male was an independent prognostic predictor of MACEs in a population with cardiovascular risks without a history of CAD. The present study suggests that increased LVMI at the time of CCTA measurement may be an independent prognostic predictor of subsequent MACEs in males as a cardiovascular risk factor without a history of CAD. Thus, our study is important in that it demonstrates that evaluating LVMI in males as a measure of cardiovascular risk may be used to predict the development of MACEs. Thus, the results of the analyses differed significantly between genders. In rat studies, females tend to maintain LV contractility in response to the degree of LVH compared to males [39] and there are differences in the degree of suppression of myocardial remodeling [40]. In humans, myocardial responsiveness to blood pressure, body size, aging, and hormones is thought to differ by gender [41], and females are less likely than males to associate myocardial natriuretic peptide with LVH. Females show less of an elevation of cardiac natriuretic peptide according to the degree of heart failure [42]. These results suggest that the reason why LVMI was not associated with MACEs in females in the MACEs group may be that females tend to have preserved myocardial structure in response to cardiac stress. Therefore, no association between MACEs and LVMI may be found in females.
Thus, although previous studies have suggested that hypertension and aging are more likely to cause LVH in males than in females, the clear cause of the difference in LVH between gender is unknown. It is also unclear whether males with DM and dyslipidemia are more likely to develop LVH than females. Similarly, no studies are known to compare which cardiovascular risks are strongly associated with LVH. In the study by Luchner et al [42], which targeted only hypertension in the general population, the prevalence of hypertension was around 50%. It is difficult to compare our study with previous studies because the target population was patients with at least one cardiovascular risk and with a high prevalence of cardiovascular risks, including hypertension. In addition, in this study, there was no significant difference between the MACEs and the non-MACEs group in terms of hypertension, dyslipidemia, and DM in both males and females. Except for LVMI, hypertension, dyslipidemia, and DM were not associated with MACEs in multivariate logistic regression analysis. Higher cardiovascular risk may have resulted in an association of LVMI. Therefore, it may be associated with MACEs in men with increased myocardial hypertrophy responsiveness to cardiovascular risk.
It has also been suggested that, in an elderly population with no history of CAD or heart failure, a history of smoking is associated with worse LVMI, LVM/volume ratio, and diastolic function [43]. It has also been suggested that a history of smoking increases the inflammatory response (C-reactive protein and interleukin-6) and cardiomyocyte damage (cardiac troponin T (hscTnT)) in the blood [44]. Thus, smoking worsens LVH and function. However, in the present study, there was no significant difference in the prevalence of smoking between the MACEs and non-MACEs groups in males. In females, the MACEs group had a significantly greater proportion of patients with a smoking history than the non-MACEs group, and smoking was also associated with MACEs in a logistic regression analysis. Thus, the theory that smoking causes LVH via inflammation was not necessarily apparent in this study.
Study limitation
This was a single-center study with a small sample size. The number of MACEs events was small for the entire population, although the patient group was at higher risk than the general population with a health examination. The study was able to evaluate patients without including those with preexisting CAD. In addition, the analysis did not consider medications to address the presence of cardiovascular risks. Future large multicenter studies will be needed.
Conclusions
Evaluation of LVMI by CCTA as a cardiovascular risk factor may be useful for predicting MACEs in males, but not females. Therefore, in the future, we need to accumulate more cases to find other factors that predict MACEs in females.
Acknowledgments
None to declare.
Financial Disclosure
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflict of Interest
We have no potential conflict of interest.
Informed Consent
All the participants were provided with the informed consent form before enrollment.
Author Contributions
Conceptualization: TT and YS; methodology: YS and KT; data collection: TT, KT, SH, YS and YK; software: YS and KT; formal analysis: TT and YS; investigation: TT and YK; writing - original draft preparation: TT; writing - review and editing: YK and TK; supervision: MS and SM. All authors have read and agreed to the published version of the manuscript.
Data Availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
| References | ▴Top |
- Mitsutake R, Miura S, Kawamura A, Saku K. Are metabolic factors associated with coronary artery stenosis on MDCT? Circ J. 2009;73(1):132-138.
doi pubmed - Mitsutake R, Miura S, Zhang B, Saku K. HDL-associated factors provide additional prognostic information for coronary artery disease as determined by multi-detector row computed tomography. Int J Cardiol. 2010;143(1):72-78.
doi pubmed - Mitsutake R, Miura S, Shiga Y, Uehara Y, Saku K. Association between hypertension and coronary artery disease as assessed by coronary computed tomography. J Clin Hypertens (Greenwich). 2011;13(3):198-204.
doi pubmed pmc - Shiga Y, Miura S, Mitsutake R, Kawamura A, Uehara Y, Saku K. Significance of serum high-density lipoprotein cholesterol levels for diagnosis of coronary stenosis as determined by MDCT in patients with suspected coronary artery disease. J Atheroscler Thromb. 2010;17(8):870-878.
doi pubmed - Shiga Y, Miura S, Mitsutake R, Yamagishi S, Saku K. Significance of plasma levels of pigment epithelium-derived factor as determined by multidetector row computed tomography in patients with mild chronic kidney disease and/or coronary artery disease. J Int Med Res. 2011;39(3):880-890.
doi pubmed - Nakamura A, Miura S, Shiga Y, Norimatsu K, Miyase Y, Suematsu Y, Mitsutake R. et al. Is Pentraxin 3 a biomarker, a player, or both in the context of coronary atherosclerosis and metabolic factors? Heart Vessels. 2014;29:603-610.
- Norimatsu K, Miura S, Suematsu Y, Shiga Y, Miyase Y, Nakamura A, Yamada M, et al. Associations between glycated albumin or hemoglobin A1c and the presence of coronary artery disease. J Cardiol. 2015;65(6):487-493.
doi pubmed - Yano M, Miura S, Shiga Y, Miyase Y, Suematsu Y, Norimatsu K, Nakamura A, et al. Association between smoking habits and severity of coronary stenosis as assessed by coronary computed tomography angiography. Heart Vessels. 2016;31(7):1061-1068.
doi pubmed - Norimatsu K, Kuwano T, Miura SI, Shimizu T, Shiga Y, Suematsu Y, Miyase Y, et al. Significance of the percentage of cholesterol efflux capacity and total cholesterol efflux capacity in patients with or without coronary artery disease. Heart Vessels. 2017;32(1):30-38.
doi pubmed - Ueda Y, Shiga Y, Idemoto Y, Tashiro K, Motozato K, Koyoshi R, Kuwano T, et al. Association between the presence or severity of coronary artery disease and pericardial fat, paracardial fat, epicardial fat, visceral fat, and subcutaneous fat as assessed by multi-detector row computed tomography. Int Heart J. 2018;59(4):695-704.
doi pubmed - Nose D, Shiga Y, Ueda Y, Idemoto Y, Tashiro K, Suematsu Y, Kuwano T, et al. Association between plasma levels of PCSK9 and the presence of coronary artery disease in Japanese. Heart Vessels. 2019;34(1):19-28.
doi pubmed - Vanhoenacker PK, Heijenbrok-Kal MH, Van Heste R, Decramer I, Van Hoe LR, Wijns W, Hunink MG. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: meta-analysis. Radiology. 2007;244(2):419-428.
doi pubmed - Schroeder S, Achenbach S, Bengel F, Burgstahler C, Cademartiri F, de Feyter P, George R, et al. Cardiac computed tomography: indications, applications, limitations, and training requirements: report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur Heart J. 2008;29(4):531-556.
doi pubmed - Rumberger JA, Sheedy PF, 3rd, Breen JF, Schwartz RS. Coronary calcium, as determined by electron beam computed tomography, and coronary disease on arteriogram. Effect of patient's sex on diagnosis. Circulation. 1995;91(5):1363-1367.
doi pubmed - Achenbach S. Detection of coronary stenoses by multidetector computed tomography: it's all about resolution. J Am Coll Cardiol. 2004;43(5):840-841.
doi pubmed - Abbara S, Chow BJ, Pena AJ, Cury RC, Hoffmann U, Nieman K, Brady TJ. Assessment of left ventricular function with 16- and 64-slice multi-detector computed tomography. Eur J Radiol. 2008;67(3):481-486.
doi pubmed - Muhlenbruch G, Das M, Hohl C, Wildberger JE, Rinck D, Flohr TG, Koos R, et al. Global left ventricular function in cardiac CT. Evaluation of an automated 3D region-growing segmentation algorithm. Eur Radiol. 2006;16(5):1117-1123.
doi pubmed - Takagi Y, Ehara S, Okuyama T, Shirai N, Yamashita H, Sugioka K, Kitamura H, et al. Comparison of determinations of left atrial volume by the biplane area-length and Simpson's methods using 64-slice computed tomography. J Cardiol. 2009;53(2):257-264.
doi pubmed - Aronow WS, Ahn C, Kronzon I, Koenigsberg M. Congestive heart failure, coronary events and atherothrombotic brain infarction in elderly blacks and whites with systemic hypertension and with and without echocardiographic and electrocardiographic evidence of left ventricular hypertrophy. Am J Cardiol. 1991;67(4):295-299.
doi pubmed - Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort. The Framingham Heart Study. Ann Intern Med. 1989;110(2):101-107.
doi pubmed - Aronow WS, Koenigsberg M, Schwartz KS. Usefulness of echocardiographic left ventricular hypertrophy in predicting new coronary events and atherothrombotic brain infarction in patients over 62 years of age. Am J Cardiol. 1988;61(13):1130-1132.
doi pubmed - Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561-1566.
doi pubmed - Verdecchia P, Angeli F, Borgioni C, Gattobigio R, de Simone G, Devereux RB, Porcellati C. Changes in cardiovascular risk by reduction of left ventricular mass in hypertension: a meta-analysis. Am J Hypertens. 2003;16(11 Pt 1):895-899.
doi pubmed - Dzau VJ. Tissue renin-angiotensin system in myocardial hypertrophy and failure. Arch Intern Med. 1993;153(8):937-942.
pubmed - Kishi S, Magalhaes TA, George RT, Dewey M, Laham RJ, Niinuma H, Friedman LA, et al. Relationship of left ventricular mass to coronary atherosclerosis and myocardial ischaemia: the CORE320 multicenter study. Eur Heart J Cardiovasc Imaging. 2015;16(2):166-176.
doi pubmed pmc - Ang DS, Pringle SD, Struthers AD. The cardiovascular risk factor, left ventricular hypertrophy, is highly prevalent in stable, treated angina pectoris. Am J Hypertens. 2007;20(10):1029-1035.
doi pubmed - Celebi AS, Yalcin H, Yalcin F. Current cardiac imaging techniques for detection of left ventricular mass. Cardiovasc Ultrasound. 2010;8:19.
doi pubmed pmc - Kaufmann BA, Min SY, Goetschalckx K, Bernheim AM, Buser PT, Pfisterer ME, Brunner-La Rocca HP. How reliable are left ventricular ejection fraction cut offs assessed by echocardiography for clinical decision making in patients with heart failure? Int J Cardiovasc Imaging. 2013;29(3):581-588.
doi pubmed - Sonaglioni A, Nicolosi GL, Granato A, Bonanomi A, Rigamonti E, Lombardo M. Influence of chest wall conformation on reproducibility of main echocardiographic indices of left ventricular systolic function. Minerva Cardiol Angiol. 2024;72(2):111-124.
doi pubmed - Klein R, Ametepe ES, Yam Y, Dwivedi G, Chow BJ. Cardiac CT assessment of left ventricular mass in mid-diastasis and its prognostic value. Eur Heart J Cardiovasc Imaging. 2017;18(1):95-102.
doi pubmed - Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, Nieman K, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8(5):342-358.
doi pubmed - Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51(3):606.
doi pubmed - Dubois D, Dubois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863-871.
- The examination committee of criteria for metabolic syndrome. Definition and criteria of metabolic syndrome. J Jpn Soc Int Med. 2005;94:794-809 (in Japanese).
- Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81(3):815-820.
doi pubmed - de Simone G, Gottdiener JS, Chinali M, Maurer MS. Left ventricular mass predicts heart failure not related to previous myocardial infarction: the Cardiovascular Health Study. Eur Heart J. 2008;29(6):741-747.
doi pubmed - Turakhia MP, Schiller NB, Whooley MA. Prognostic significance of increased left ventricular mass index to mortality and sudden death in patients with stable coronary heart disease (from the Heart and Soul Study). Am J Cardiol. 2008;102(9):1131-1135.
doi pubmed pmc - Abdi-Ali A, Miller RJH, Southern D, Zhang M, Mikami Y, Knudtson M, Heydari B, et al. LV mass independently predicts mortality and need for future revascularization in patients undergoing diagnostic coronary angiography. JACC Cardiovasc Imaging. 2018;11(3):423-433.
doi pubmed - Weinberg EO, Thienelt CD, Katz SE, Bartunek J, Tajima M, Rohrbach S, Douglas PS, et al. Gender differences in molecular remodeling in pressure overload hypertrophy. J Am Coll Cardiol. 1999;34(1):264-273.
doi pubmed - Tamura T, Said S, Gerdes AM. Gender-related differences in myocyte remodeling in progression to heart failure. Hypertension. 1999;33(2):676-680.
doi pubmed - Escudero EM, Orlowski A, Diaz A, Pinilla OA, Ennis IL, Aiello EA. Gender differences in cardiac left ventricular mass and function: Clinical and experimental observations. Cardiol J. 2014;21(1):53-59.
doi pubmed - Luchner A, Brockel U, Muscholl M, Hense HW, Doring A, Riegger GA, Schunkert H. Gender-specific differences of cardiac remodeling in subjects with left ventricular dysfunction: a population-based study. Cardiovasc Res. 2002;53(3):720-727.
doi pubmed - Nadruz W, Jr., Claggett B, Goncalves A, Querejeta-Roca G, Fernandes-Silva MM, Shah AM, Cheng S, et al. Smoking and cardiac structure and function in the elderly: the ARIC study (Atherosclerosis Risk in Communities). Circ Cardiovasc Imaging. 2016;9(9):e004950.
doi pubmed pmc - Gottdiener JS, Buzkova P, Kahn PA, DeFilippi C, Shah S, Barasch E, Kizer JR, et al. Relation of cigarette smoking and heart failure in adults >/=65 years of age (From the Cardiovascular Health Study). Am J Cardiol. 2022;168:90-98.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


