| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Short Communication
Volume 15, Number 3, June 2024, pages 198-204
Is Type 2 Diabetes Mellitus an Independent Risk Factor for Mortality in Hypertrophic Cardiomyopathy?
Said Hajoulia, c, Adam Belcherb, Frank Annieb, Ahmad Elasherya
aDepartment of Cardiology, Charleston Area Medical Center, Charleston, WV, USA
bInstitute for Academic Medicine, Charleston Area Medical Center, Charleston, WV, USA
cCorresponding Author: Said Hajouli, Department of Cardiology, Charleston Area Medical Center, Charleston, WV 25304, USA
Manuscript submitted May 1, 2024, accepted May 23, 2024, published online June 25, 2024
Short title: T2DM and HCM
doi: https://doi.org/10.14740/cr1659
| Abstract | ▴Top |
Background: The mortality rate of hypertrophic cardiomyopathy (HCM) has decreased between 1999 and 2020. The risk factors for sudden cardiac death (SCD) in HCM were updated in the American Heart Association (AHA)/American College of Cardiology Foundation (ACCF) 2020 guidelines by adding new risk factors, like the late gadolinium enhancement on cardiac magnetic resonance imaging (MRI). Type 2 diabetes mellitus (T2DM) is a major risk factor for most cardiac diseases; however, it is not included in these guidelines due to a lack of strong evidence of a correlation between T2DM and mortality in HCM. Therefore, we sought to investigate if T2DM increases the 5-year risk rate for adverse outcomes, such as heart failure and all-cause mortality in patients with HCM.
Methods: We collected patient data from January 1, 2018, to March 1, 2023, using the TriNetX database. The sample included 80,502 individuals with HCM, then divided into two cohorts based on the absence (58,573; cohort 1) or presence (15,296; cohort 2) of T2DM. The two matched groups then underwent survival and risk analyses for all-cause mortality or the first incidence of heart failure diagnosis within 5 years from the point in time when the selection criteria were first met.
Results: We found a statistically significant increase in all-cause mortality and new-onset heart failure in HCM patients with diabetes compared to those without diabetes after adjusting for major risk factors.
Conclusions: This is one of the largest retrospective cohort studies that examined the correlation between T2DM and adverse outcomes in patients with HCM. This underlines the need for future prospective studies investigating the effects of T2DM on HCM outcomes.
Keywords: All-cause mortality; Diabetes mellitus; Hypertrophic cardiomyopathy; Heart failure
| Introduction | ▴Top |
Hypertrophic cardiomyopathy (HCM) is a cardiac muscular disease characterized by abnormal left ventricle (LV) wall thickening and atypical myocyte organization [1]. The estimated prevalence of HCM is 0.2% to 0.3% in the adult population, with approximately 60% of those cases showing evidence of a familial disease [2]. It is not commonly found in children and adolescents, given its incidence rate of approximately 1.3:100,000 in patients aged ≤ 17 years old; however, it does carry a significant risk of sudden cardiac death (SCD) in young athletes, with around 40% of cases being linked to HCM [3, 4]. Manifestations of HCM can range from asymptomatic to SCD. The risks of SCD were described in the 2020 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines, which include: LV wall thickness of ≥ 30 mm, unexplained syncope, family history of SCD from HCM, left ventricular ejection fraction (LVEF) < 50%, LV apical aneurysm of any size, nonsustained ventricular tachycardia (NSVT) on an ambulatory cardiac monitor, and > 15% late gadolinium enhancement (LGE) on cardiovascular magnetic resonance imaging CMR [5].
Type 2 diabetes mellitus (T2DM) is a major risk factor for multiple cardiovascular disorders, including coronary artery disease (CAD), myocardial infarction (MI), conduction disorders and arrhythmia, stroke, diabetic cardiomyopathy, and heart failure. The impact of T2DM on HCM outcomes has not been extensively studied thus far. Therefore, we sought to determine if a correlation exists between T2DM and worsened HCM outcomes using deidentified patient data collected from the TriNetX database.
| Materials and Methods | ▴Top |
Patient data and cohort criteria
The authors received permission to conduct this test from the Charleston Area Medical Center Internal Review Board.
The patient data were collected on May 20, 2024, for this study from the TriNetX Research Network consisting of deidentified, per Section §164.514(a) of the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule, electronic health records (diagnoses, medications, procedures, labs, etc.) from a national and international healthcare organization. In this study, 79 healthcare organizations, consisting of 102,899,907 patients, were used. The TriNetX platform complies with the HIPAA and general data protection regulations. Patient data were gathered through a deidentified database compliant with HIPAA regulations. The data were restricted to HCM (International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) I42.1 and I42.2) patients aged 18 to 90 and records dated between January 1, 2018, and March 1, 2023. Furthermore, patients with mitral and/or aortic valve stenosis (ICD-10-CM I35.0, I06.0, Q25.3, I08.0, and Q23.0) were excluded from the study. In total, there were 73,869 HCM patients included in the study that were then separated into two cohorts based on the absence (cohort 1) or presence (cohort 2) of T2DM (ICD-10-CM E11). Additional exclusion criteria for cohort 1 were the presence of any type of diabetes (ICD-10-CM E08-E13), insulin use (VA HS501), sulfonylureas (Anatomical Therapeutic Chemical codes (ATC) A10BB), alpha glucosidase inhibitors (ATC A10BF), thiazolidinediones (ATC A10BG), dipeptidyl peptidase 4 inhibitors (ATC A10BH), sodium-glucose cotransporter 2 inhibitors (ATC A10BK), or HbA1C ≥ 6.4% (TriNetX Curated Values 9037). Out of these patients with HCM, 58,573(79%) did not have T2DM (cohort 1) and 15,296 (21%) had T2DM (cohort 2).
Baseline characteristics
Descriptive statistics were presented as frequencies with percentages for categorical variables and mean and standard deviation for continuous measures of association between groups. Baseline characteristics were compared using Pearson’s test for categorical variables and independent sample t-tests for continuous variables.
Propensity score matching (PSM)
TriNetX’s PSM algorithm was used to account for differences in the groups’ baseline characteristics. The PSM algorithm calculates covariate values for each patient and then places each into a matrix. Afterward, it performs a logistic regression on the combined matrices to generate a propensity score for each patient. The patients are then matched using greedy nearest neighbor matching with a caliper of 0.1 pooled standard deviations to create a subset cohort of best-matched patients. The covariates used for our analyses were: age, sex, chronic obstructive pulmonary disease (COPD, ICD-10-CM J44), primary hypertension (HTN, ICD-10-CM I10), pulmonary hypertension (ICD-10-CM I27.20), body mass index (BMI, TriNetX curated codes (TNX) 9083), family history of SCD (ICD-10-CM Z82.41), B-type natriuretic peptide levels (BNP, TNX 9003), estimated glomerular filtration rate (eGFR, TNX 8001), nicotine dependence (ICD-10-CM Z87.891), use of statins (VA CV350, anticoagulant therapy (VA BL110), syncope (ICD-10-CM R55), CAD (ICD-10-CM I25.10), chronic kidney disease (CKD, ICD-10-CM N18), end-stage renal disease (ESRD, ICD-10-CM N18.6), atrial fibrillation (Afib)/flutter (ICD-10-CM I48), hyperlipidemia (HLD, ICD-10-CM E78.5), presence of implantable cardioverter-defibrillator device (ICD-10-CM Z95.810), ventricular tachycardia (VT, ICD-10-CM I47.2), ventricular fibrillation (Vfib, ICD-10-CM I49.0), beta blockers (VA CV100), antiarrhythmics (VA CV300), angiotensin-converting enzyme inhibitors (ACEI, VA CV800), angiotensin II inhibitors (ARBs, VA CV805), platelet inhibitors (VA BL117), left atrial diameter anterior-posterior systole by two-dimensional ultrasound (2-D US, logical observation identifiers names and codes (LOINC) 29468-6), systolic blood pressure (TNX 9085), left ventricle ejection fraction (LVEF, TNX 2003), thickness (length) of interventricular spetum (LOINC 99528-2), and surgical intervention of the mitral valve (Current Procedural Terminology codes (CPT) 1006140). Summarized cohort characteristics after PSM can be found in Table 1. After PSM, there were 14,279 patients in each cohort.
 Click to view | Table 1. Baseline Characteristics After Propensity Score Matching |
Measures of association
The measures of association analysis are used to compare the ratio of patients with the selected outcome (death or new-onset heart failure). The analysis calculates risk (number of patients with outcome/number of patients in the cohort), risk difference (cohort 1 risk - cohort 2 risk), risk ratio (cohort 1 risk/cohort 2 risk), and odds ratio (cohort 1 odds/cohort 2 odds).
Kaplan-Meir survival analysis
The survival analysis predicts the probability of an outcome (death or new-onset heart failure) during the set time period (5 years). Data censoring was applied to account for patients who exited the cohort before the end of the time period, and, therefore, the last point of data in their chart was used. Additionally, the data were further analyzed using the log-rank test, hazard ratio, and test for proportionality.
| Results | ▴Top |
The primary objective of this study was to compare all-cause mortality and new-onset heart failure diagnosis 5-year risk rates between patients with HCM (cohort 1: 58,573) and patients with HCM and diabetes (cohort 2: 15,296). To this extent, risk and survival analyses were conducted on the propensity-matched cohorts (14,279 patients each) within the selected 5-year timeframe (January 1, 2018, to March 1, 2023).
Risk analysis
Risk analyses between cohort 1 and cohort 2 showed that 1,338 (9.37%) patients without diabetes and 1,948 (13.64%) patients with diabetes were deceased 5 years after the index event. The risk difference between cohort 1 and cohort 2 was -4.27% with a 95% confidence interval (CI) of (-5.01%, -3.53%), z-score of -11.31, and P value < 0.0001. The risk ratio was 0.69 with a 95% CI of (0.64, 0.73), and the odds ratio was 0.65 with a 95% CI of (0.61, 0.71). Risk analyses are summarized in Table 2. Additionally, risk analyses were conducted between the cohorts to determine the 5-year risk rate of new-onset heart failure. For these analyses, 10,474 and 9,246 patients were in cohort 1 and 2, respectively, after PSM. The 5-year risk rate for cohort 1 developing new-onset heart failure was 12.36% (1,295 with outcome) and 15.85% (1,465 with outcome) for cohort 2. The risk difference was -3.48% with a 95% CI of (-4.46%, -2.51%), z-score of -7.03, and P value of < 0.0001. The risk ratio was 0.78 with a 95% CI of (0.73, 0.84), and the odds ratio was 0.75 with a 95% CI of (0.69, 0.81). Three thousand eight hundred and five patients in cohort 1 and 5,033 patients in cohort 2 were excluded from the results because they had a diagnosis of heart failure prior to the time window. These results are summarized in Table 3.
 Click to view | Table 2. Outcome of Death |
 Click to view | Table 3. Outcome of New-Onset Heart Failure |
Kaplan-Meier survival analysis
Kaplan-Meier survival curve analysis showed that survival probability against the outcome of death was 85.51% for cohort 1 and 79.87% for cohort 2 (Fig. 1a). Log-rank test (χ2 = 149.38, degree of freedom (df) = 1) showed statistical significance with a P value < 0.0001. Survival analysis against the outcome of new-onset heart failure was 79.60% for cohort 1 and 74.47% for cohort 2 (Fig. 1b). Log-rank test (χ2 = 71.96, df = 1) showed statistical significance with a P value of < 0.0001.
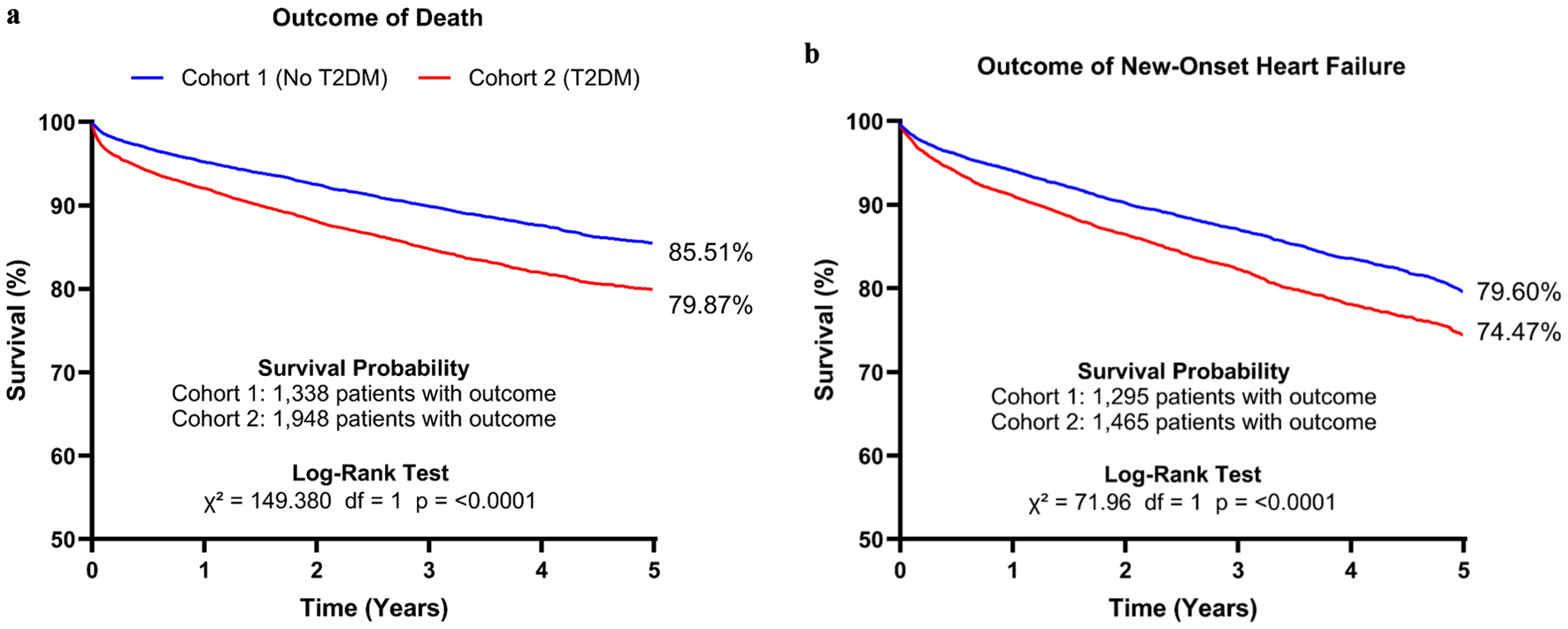 Click for large image | Figure 1. Kaplan-Meier survival curve analysis and log-rank test against the outcome of death (a) for cohort 1 and cohort 2 over the course of 5 years after the index event. Analyses were also conducted against the outcome of heart failure over the same period (b). T2DM: type 2 diabetes mellitus. |
| Discussion | ▴Top |
This is the first large retrospective cohort study using the TriNetX database to evaluate the impact of T2DM on HCM outcomes. We found that T2DM was an independent risk factor in HCM patients, given its statistically significant higher all-cause-mortality rate and heart failure incidences compared to HCM patients without T2DM after adjusting for multiple variables, including age, sex, race, HTN, HLD, COPD, nicotine dependence, CAD, CKD, ESRD, arrhythmias, pulmonary HTN, and medications, among other things.
HCM is mainly a genetic structural heart disease that affects the myocardium, and it is typically inherited in an autosomal dominant pattern with a prevalence rate of approximately 0.2-1.4% in the general population [1, 2]. Nonfamilial HCM is described as unexplained LV hypertrophy of > 15 mm, normal LV chambers, and the absence of family history or clinically significant genetic variants [6]. Ingles et al [6] sought to profile nonfamilial HCM by evaluating 413 patients from a specialized HCM clinic from 2002 to 2015. Of these patients, 40% of the HCM probands were identified as nonfamilial HCM with a mean age of 61. Most of these patients had a benign disease course with fewer incidences of SCD, HF, and stroke compared to the patients with familial or genetic HCM [6]. In our study, the mean age at index was 62 years old which closely matches the mean age found in the study by Ingles et al [6].
There is an association between common cardiovascular risk factors, such as HTN, T2DM, and obesity, and the severity of HCM, regardless of genetics or family history [7]. For this study, we investigated the effect of T2DM on all-cause mortality and new-onset heart failure diagnosis in patients with HCM. T2DM is a multisystem disease with known significant effects on the cardiovascular system [8]. There have been several studies investigating potential mechanisms, including inflammation, oxidative stress, and endothelial dysfunction, in which diabetes and other metabolic disorders enhance the risk or severity of HCM [9].
In this large cohort study, we demonstrated that HCM patients with T2DM have a higher mortality rate and incidence of new-onset heart failure than those without T2DM, independently of other cardiovascular risk factors in the 5 years following initial diagnosis. Given this, our study highlights the need for more investigation to further assess the relationship between T2DM and HCM outcomes. Vigilant monitoring and treatment of HCM patients with T2DM could help reduce this increase in new-onset heart failure and all-cause mortality.
One strength of this study is the large sample size. We included 73,869 HCM patients, where 58,573 (79.3%) did not have T2DM and 15,296 (20.7%) did. To control confounding factors, we used PSM to control for multiple cardiovascular risk factors, relevant bloodwork results, and medications. We used standardized and TriNetX curated, when applicable, medical codes to ensure proper selection of PSM inputs. Kaplan-Meier survival and risk analyses on the balanced cohorts showed a statistically significant increase in risk of death in a 5-year period after the index event in patients with HCM and T2DM (13.64%) compared to patients with only HCM (9.37%). Additionally, the survival probability for patients with HCM and T2DM (79.87%) at 5-year post-index was lower than HCM patients without T2DM (85.51%).
Our data reinforce data from previous studies demonstrating that T2DM was associated with poorer outcomes in patients with HCM [10-13]. For example, a study in 2022 by Lee et al [11] analyzed adverse outcome risk between HCM patients with and without T2DM using data from the Korean National Health Insurance Service database. Their study found that diabetes was associated with a 15% increase in HF after matching. However, it was only associated with higher all-cause mortality in unmatched cohorts. Their study could not adjust for hemoglobin A1c (HbA1c) levels, EF, or hypertrophy between the groups. Our study also benefited from a much larger patient data database (14,279 vs. 1,118 after matching).
The limitations in our study primarily center on the potential for errors or differences in patient charting amongst healthcare systems. TriNetX curates the data to ensure proper labeling; however, patient records may be incomplete or inaccurate from the source. We sought to decrease these errors by including HbA1c, insulin use, and other blood glucose-lowering medications in the selection criteria, in addition to diagnosis with diabetes. Additionally, the large number of patients included in the analysis reduces the impact of implicit errors. Other limitations include a lack of data on the specific causes of death and the inability to directly compare cardiac imaging.
Conclusions
T2DM is an independent risk factor for all-cause mortality and new-onset heart failure in patients with HCM. Our study showed a statistically significant increase in all-cause mortality risk and incidence of new-onset heart failure at 5 years post-index. Considering these findings, future large-cohort studies investigating specific causes of death, such as SCD, in patients with T2DM and HCM would be greatly beneficial.
Acknowledgments
None to declare.
Financial Disclosure
No funding was provided for this study.
Conflict of Interest
The authors state that they have no conflict of interest to declare.
Informed Consent
This retrospective secondary analysis of existing, deidentified patient data is exempt from informed consent.
Author Contributions
Dr. Said Hajouli developed the idea and wrote the initial drafts of the manuscript. Dr. Adam Belcher and Dr. Frank Annie provided research support, data analysis, and text revisions. Dr. Ahmad Elashery approved the manuscript and provided guidance through his supervisory role.
Data Availability
Deidentified patient data were provided by the TriNetX (http://www.trinetx.com) federated data network. Access to the data is available for healthcare research after obtaining license approval.
Abbreviations
HCM: hypertrophic cardiomyopathy; SCD: sudden cardiac death; T2DM: type 2 diabetes mellitus; LV: left ventricle; LVEF: left ventricular ejection fraction; NSVT: non-sustained ventricular tachycardia; CAD: coronary artery disease; MI: myocardial infarction; PSM: propensity score matching; ICD-10-CM: International Classification of Diseases, 10th Revision, Clinical Modification; LOINC: logical observation identifiers names and codes; VA: VA national formulary codes; ATC: Anatomical Therapeutic Chemical codes; CPT: Current Procedural Terminology codes; TNX: TriNetX curated codes; COPD: chronic obstructive pulmonary disease; BMI: body mass index; CKD: chronic kidney disease; ESRD: end-stage renal disease; Afib: atrial fibrillation; Vfib: ventricular fibrillation; HLD: hyperlipidemia; HTN: hypertension; VT: ventricular tachycardia; ICD: implantable cardioverter-defibrillator device; ACEI: angiotensin-converting enzyme inhibitors; ARBs: angiotensin II receptor blockers; BP: blood pressure; BNP: B-type natriuretic peptide
| References | ▴Top |
- Mohrman DE, Heller LJ. Cardiovascular function in pathological situations. In: Cardiovascular Physiology, 9e. New York, NY: McGraw-Hill Education; 2018.
- Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017;121(7):749-770.
doi pubmed pmc - Nandi D, Hayes EA, Wang Y, Jerrell JM. Epidemiology of pediatric hypertrophic cardiomyopathy in a 10-year medicaid cohort. Pediatr Cardiol. 2021;42(1):210-214.
doi pubmed - Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. 2009;119(8):1085-1092.
doi pubmed - Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142(25):e558-e631.
doi pubmed - Ingles J, Burns C, Bagnall RD, Lam L, Yeates L, Sarina T, Puranik R, et al. Nonfamilial hypertrophic cardiomyopathy: prevalence, natural history, and clinical implications. Circ Cardiovasc Genet. 2017;10(2):e001620.
doi pubmed - Lopes LR, Losi MA, Sheikh N, Laroche C, Charron P, Gimeno J, Kaski JP, et al. Association between common cardiovascular risk factors and clinical phenotype in patients with hypertrophic cardiomyopathy from the European Society of Cardiology (ESC) EurObservational Research Programme (EORP) Cardiomyopathy/Myocarditis registry. Eur Heart J Qual Care Clin Outcomes. 2022;9(1):42-53.
doi pubmed pmc - Wong ND, Sattar N. Cardiovascular risk in diabetes mellitus: epidemiology, assessment and prevention. Nat Rev Cardiol. 2023;20(10):685-695.
doi pubmed - Salvatore T, Pafundi PC, Galiero R, Albanese G, Di Martino A, Caturano A, Vetrano E, et al. The diabetic cardiomyopathy: the contributing pathophysiological mechanisms. Front Med (Lausanne). 2021;8:695792.
doi pubmed pmc - Jex N, Chowdhary A, Thirunavukarasu S, Procter H, Sengupta A, Natarajan P, Kotha S, et al. Coexistent diabetes is associated with the presence of adverse phenotypic features in patients with hypertrophic cardiomyopathy. Diabetes Care. 2022;45(8):1852-1862.
doi pubmed pmc - Lee HJ, Kim HK, Kim BS, Han KD, Rhee TM, Park JB, Lee H, et al. Impact of diabetes mellitus on the outcomes of subjects with hypertrophic cardiomyopathy: A nationwide cohort study. Diabetes Res Clin Pract. 2022;186:109838.
doi pubmed - Mekhaimar M, Al Mohannadi M, Dargham S, Al Suwaidi J, Jneid H, Abi Khalil C. Diabetes outcomes in heart failure patients with hypertrophic cardiomyopathy. Front Physiol. 2022;13:976315.
doi pubmed pmc - Wang S, Cui H, Ji K, Song C, Ren C, Guo H, Zhu C, et al. Impact of type 2 diabetes mellitus on mid-term mortality for hypertrophic cardiomyopathy patients who underwent septal myectomy. Cardiovasc Diabetol. 2020;19(1):64.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


