| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website http://www.cardiologyres.org |
Case Report
Volume 9, Number 4, August 2018, pages 258-263
Technical Considerations in Transradial Unprotected Left Main Stem Rotational Atherectomy-Assisted and IVUS-Guided Percutaneous Coronary Intervention Using the 7.5F Eaucath Sheathless Guiding Catheter System
George Kassimisa, b, c, Nicholas Weighta, Nestoras Kontogiannisa, Tushar Rainaa
aDepartment of Cardiology, Cheltenham General Hospital, Gloucestershire Hospitals NHS Foundation Trust, Cheltenham, UK
bSecond Department of Cardiology, Hippokration Hospital, Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece
cCorresponding Author: George Kassimis, Cheltenham General Hospital, Gloucestershire Hospitals NHS Foundation Trust, Cheltenham GL53 7AN, UK
Manuscript submitted June 6, 2018, accepted June 15, 2018
Short title: Sheathless Radial Left Main Rotablation
doi: https://doi.org/10.14740/cr740w
| Abstract | ▴Top |
Rotational atherectomy-assisted percutaneous coronary intervention (PCI) on unprotected left main stem (LMS) bifurcation lesions is technically challenging. Intravascular ultrasound (IVUS) has become a standard part of the PCI procedure for the treatment of LMS disease. There is limited experience in performing these cases via a transradial approach using a sheathless guiding catheter (SGC) system. We report a case of a symptomatic octogenarian patient with restrictive angina and significant LMS bifurcation disease, who was successfully treated transradially with the use of the 7.5F Eaucath SGC system and we describe the technical challenges encountered with this strategy.
Keywords: Radial; Sheathless guiding catheter system; Percutaneous coronary intervention; Rotational atherectomy; Left main stem bifurcation; Intravascular ultrasound
| Introduction | ▴Top |
Significant left main stem (LMS) disease has been traditionally treated with respect as the LMS provides blood supply to at least two-thirds of the left ventricle [1], since prognosis after diagnosis of LMS obstruction is poor without treatment and because percutaneous coronary intervention (PCI) to the LMS usually involves a major bifurcation [2]. These historical data and technical challenges resulted in coronary bypass grafting (CABG) being the standard treatment of LMS disease especially because of the excellent results achieved using the left internal mammary artery graft to the left anterior descending artery (LAD) [3].
PCI for unprotected LMS stenosis is now considered an acceptable alternative to CABG. Data from several preliminary studies [4-6] and the results from the randomized SYNTAX (Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) trial sub-study [7] have supported the use of PCI for the treatment of unprotected LMS stenosis as a valid alternative option in selected patient populations.
CABG maintains a class I indication across all anatomical subgroups. It is interesting to note that PCI assumes a higher position in the ESC/EACTS guidelines with a class I recommendation in patients with a low SYNTAX score (≤ 22) and IIa recommendation in patients with an intermediate SYNTAX score (23 - 32) [8]. In contrast, the ACCF/AHA/SCAI guidelines for PCI support a class IIa recommendation for treatment of unprotected LMS disease in patients with a low SYNTAX score and a class IIb recommendation in patients with an intermediate SYNTAX score. Both guidelines currently agree on the superiority of CABG for the treatment of LMS disease in patients with a high SYNTAX score of > 32 [9]. Since the publication of the findings of the recent EXCEL (Evaluation of XIENCE Everolimus Eluting Stent Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization) and NOBLE (Nordic-Baltic-British Left Main Revascularization Study) trials, there has been a renewed interest in PCI for the treatment of LMS disease in patients with low-intermediate SYNTAX score [10, 11].
PCI on the distal LMS represent a particularly challenging and high-risk subset, as a bifurcation stenting strategy may be required for an optimal result [12]. Large- or very large-bore guide catheters (GCs) (≥ 7F) are usually necessary for addressing such lesions. An added layer of complexity arises if adequate lesion preparation with high-speed rotational atherectomy (HSRA) is required for calcific lesions, which can be safely performed through the radial approach, with less bleeding complications and shorter in-hospital stay [13-16]. However, the size of the radial artery (RA) limits the maximum dimensions of potential GCs to 6F in most patients [17]. It is now possible to push the limits of transradial complex PCI such that 7F GCs can be advanced and manipulated through a Slender approach [18]. Nevertheless, not all RAs will allow such interventions: some cannot be cannulated using a sheath-based access and others will occlude thereafter [19].
The inability in some patients of using a 7F sheath-based approach restricts the radial operator to a 6F GC system and limits the maximum size of rota burr to 1.75 mm, which can usually be delivered but will not allow sufficient contrast injection for angiography. Therefore, many operators restrict their choice of rota burr to 1.5 mm when using a 6F guide for safety reasons and better visualization, and HSRA has continued to be performed using 7F or 8F guides via the femoral artery.
Sheathless guiding catheter (SGC) systems allow the passage of large-bore catheters with smaller overall diameters at the arterial insertion site, as there is no need for a sheath [20]. Using a 7.5F SGC system, complex PCI can be safely performed as we previously described [15, 16]. These guides have an external diameter smaller than a sheath used for a 6F GC (2.49 mm versus 2.70 mm, respectively), but a significantly larger internal lumen (2.06 mm versus 1.78 mm, respectively) (Fig. 1). However, there are only few published data of its use in LMS bifurcation HSRA-assisted PCI. We report a case of an octogenarian patient with significant peripheral arterial disease (PAD) and severe calcific distal LMS stenosis with symptoms refractory to maximal medical therapy, who was successfully treated via a radial approach with the use of the 7.5F Eaucath SGC system and we analyze the technical challenges encountered with this approach.
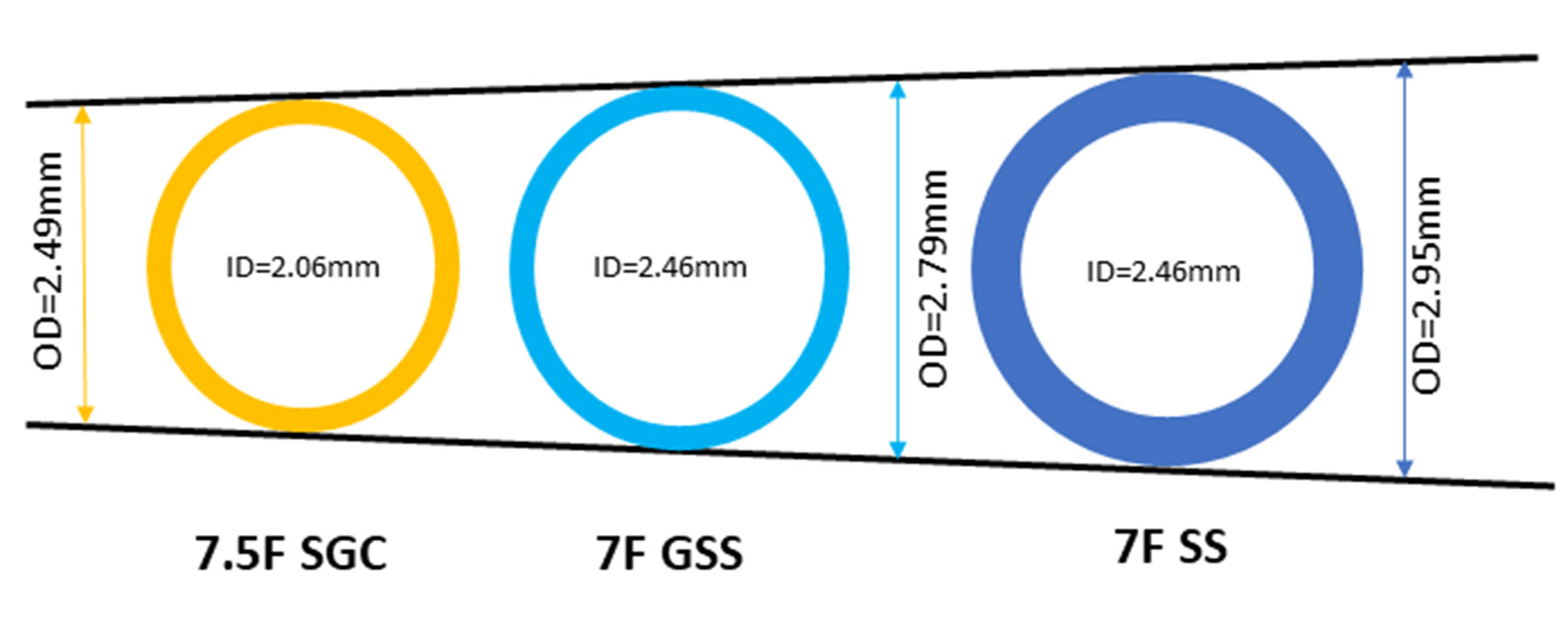 Click for large image | Figure 1. Internal and outer diameters of 7F standard sheath, 7.5F Eaucath sheathless guiding catheter and 7F glidesheath slender sheath. SS, standard sheath; SGC, sheathless guiding catheter; GSS, glidesheath slender sheath. |
| Case Report | ▴Top |
An 83-year-old male with a 6-month history of stable angina CCS class III was admitted in our center. Past medical history included significant PAD, hypertension and chronic kidney dysfunction stage 3a with eGFR 53.8 mL/min/1.73 m2. He was on maximal tolerated optimal medical therapy and TRA demonstrated severe ostial and mid LAD disease and at least moderate calcified LMS disease in a right coronary artery (RCA) unobstructed dominant system. The ostium of the circumflex (LCx) was angiographically free of disease, with a mild lesion just before the bifurcation with a relatively big first obtuse marginal (Fig. 2a and b).
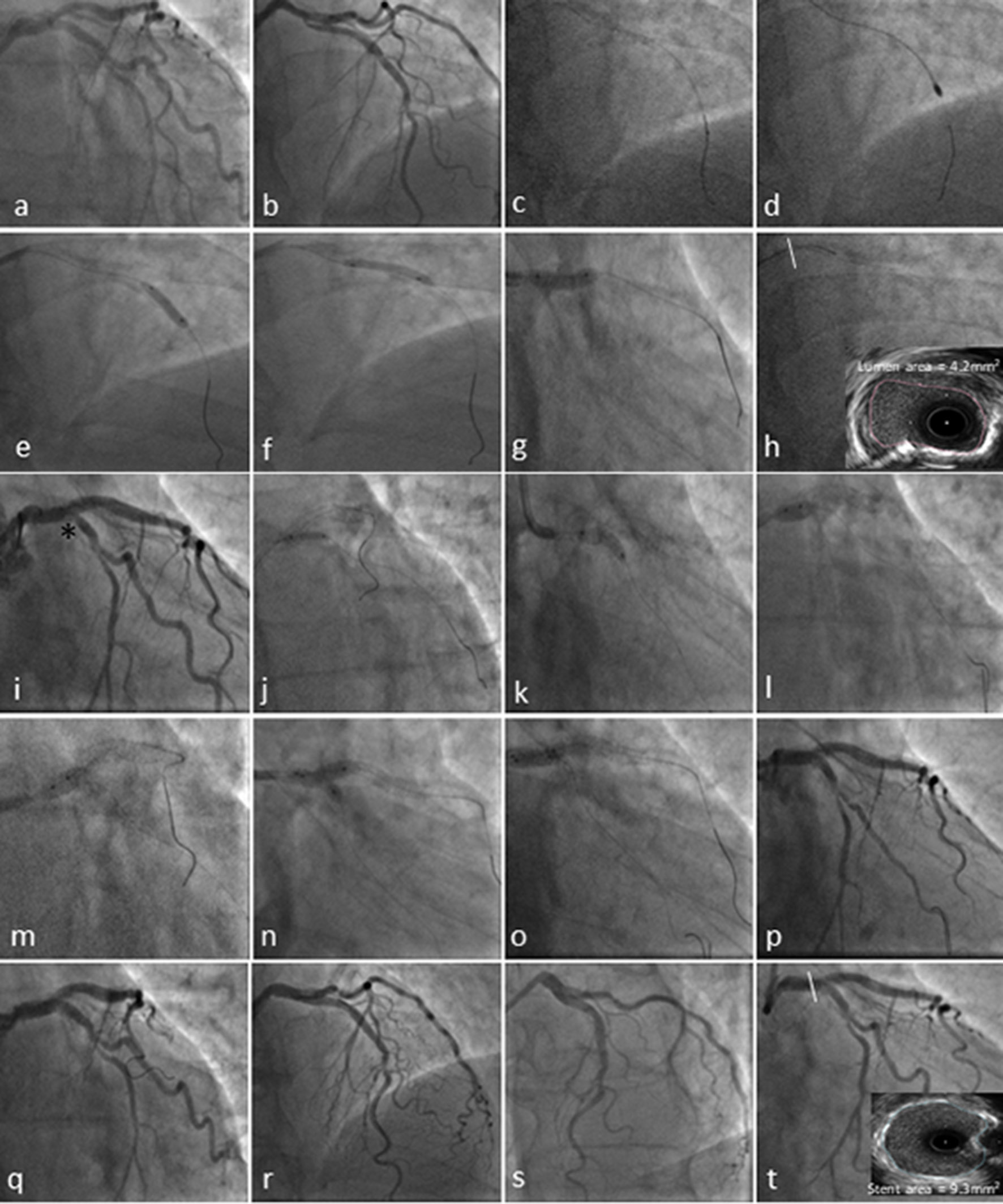 Click for large image | Figure 2. Coronary angiography and transradial percutaneous coronary intervention using the 7.5F Eaucath sheathless guide catheter. Coronary angiography demonstrated severe ostial and mid left anterior descending (LAD) disease and at least moderate distal calcified left main stem (LMS) (1,1,0 Medina) disease in a right coronary artery (RCA) unobstructed dominant system (not shown). The circumflex (LCx) ostium was angiographically free of disease, with a mild lesion just before the bifurcation with a relatively big first obtuse marginal (a and b). The LAD was wired with a BMW wire and exchanged using a Finecross microcatheter with the rotawire (2c). A 1.75-mm burr was used to ablate LMS and LAD lesions (2d), and predilated with a 3.0 × 15 mm and 3.5 × 15 mm NC Quantum Apex balloons (e-g). The pullback IVUS images showed significant calcified disease in the distal LMS with a cross-sectional luminal area of 4.2 mm2 (2h). The mid LAD lesion was stented with 3.5 × 16 mm Promus Premier and a second stent Promus Premier 4.0 × 28 mm was implanted from LMS to LAD across the Cx and post-dilated with a 4.5 NC Quantum Apex balloon (i). Due to the threatened morphology of the LCx ostium and an FFR 0f 0.78 following LMS/LAD stenting (i*), we decided to convert the strategy to a two-stent bifurcation culotte procedure. We crossed the LCx with a Pilot 50 wire and opened the struts of LMS to Cx with a semi-compliant balloon Sprinter Legend 2.0 × 12 mm (j). A 3.5 × 12 mm Promus Premier was implanted from LMS to Cx (k) and then potted the LMS with a 4.5 × 6 mm NC Quantum Apex (l and m). A final kissing balloon inflation was performed with a 3.5 × 12 mm NC balloon in the LAD and 3.25 × 12 mm NC balloon in Cx (n and o). A good angiographic result was obtained in LMS, LAD and Cx (p-s) with a post-PCI IVUS in distal LMS demonstrating a stent area of 9.3 mm2. |
The SYNTAX score I was 25 and II for PCI 37.1 and for CABG 46.1 with 4-year mortality 12% and 24%, respectively. He subsequently underwent a dobutamine stress-echo which demonstrated significant inducible LAD ischemia, with no ischemia of the Cx territory. He was discussed within the Heart Team and was felt to be suitable for transradial intravascular ultrasound (IVUS)-guided LMS PCI. Technical considerations included the need for a bifurcation stenting strategy and HSRA for the severely calcified LMS and LAD lesions.
Vascular access
Transfemoral access was not an option due to the significant PAD. A 7F introducer Glidesheath Slender Sheath (GSS; Terumo Corp., Tokyo, Japan) is not available in our center; therefore, a sheathless approach was applied. Right RA access was obtained with a 6F sheath (Radifocus®; Terumo Medical Corp., Piscataway, NJ, USA) that is used to insert a standard J-tipped 150 cm 0.035-inch diameter exchange wire (Terumo Corp.). Normal saline (10 mL) with 250 µg of nitroglycerine was injected into the RA through the introducer sheath to prevent radial spasm. The sheath was then exchanged for the SGC over the standard 150 cm J-tipped 0.035-inch wire.
The sheathless Eaucath guiding catheter system
An SPB 3.5 SGC Eaucath 7.5F (ASAHI Eaucath SGC; Vascular Perspectives Ltd, Manchester, UK) was used with good catheter support. The SGC is composed of two parts, a hydrophilic catheter and a central dilator. The central dilator was inserted into the catheter and locked in place. The SGC with the central dilator was advanced along the 0.035-inch wire to the proximal ascending thoracic aorta. The central dilator and 0.035-inch wire were then removed. The SGC has a slightly translucent tip and care is necessary with initial manipulation, particularly when calcified LMS disease has been documented. Occasionally, with this SPB SGC design the initial engagement can be problematic and the guide can dislodge, pulling the guidewire out during deep inspiration. The risk of this may be diminished by waiting at least 3 or 4 min with the white introducer sheath inside the catheter prior to its introduction into the patient.
HSRA
Following set-up shots (Fig. 2a and b), there was evidence of LMS 1,1,0 Medina bifurcation lesion and the LAD was wired with a BMW wire (Abbott Vascular, Northern California, USA). Given the extensive LMS and LAD calcific burden on angiography, a decision was made to perform plaque modification with HSRA, using the Rotablator® rotational atherectomy system (Boston Scientific, Natick, MA, USA). The initial wire was exchanged using a Finecross (Terumo, Japan) (Fig. 2c). A 1.75 mm burr was selected to reach a burr/vessel ratio approaching 0.6 with an intention to use only a single burr to ablate LMS and LAD plaque and facilitate the passage of further devices (Fig. 2d). HSRA speed was set at 150.000 rotations per minute. In our center, HSRA is usually performed in conjunction with an intracoronary infusion of a “cocktail” containing verapamil, heparin and nitroglycerine with burr runs < 20 s in duration to avoid burr deceleration. Two passes of rotablation were performed into the LAD and the LAD/LMS lesions were predilated with a 3.0 × 15 mm and 3.5 × 15 mm NC Quantum Apex balloons (Boston Scientific), respectively (Fig. 2e-g).
Intravascular imaging with IVUS
The pullback IVUS (Boston Scientific, Natick, MA, USA) images showed significant calcified disease in the mid LAD, into the ostium of the LAD and distal LMS. The cross-sectional luminal area at the distal LMS was noted to be 4.2 mm2 (Fig. 2h), mandating the necessity of revascularization [1, 21]. Significant calcification of the LMS was noted with a calcific arc of 120° (Fig. 2h). IVUS examination of the ostium of the circumflex following pullback from the LAD demonstrated only mild plaque burden; therefore, the initial LMS PCI strategy was for provisional stenting [22, 23]. The mid LAD lesion was stented with 3.5 × 16 mm Promus Premier (Boston Scientific) and post dilated with a 3.5 × 12 mm NC Quantum Apex balloon. A second stent Promus Premier 4.0 × 28 mm was implanted from LMS to LAD across the Cx and post-dilated with a 4.5 NC Quantum Apex balloon (Fig. 2i). Due to the threatened morphology of the LCx ostium with carina shift following LMS/LAD stenting (Fig. 2i*), we performed a pressure wire study of the jailed LCx and the FFR was 0.78; therefore, we decided to convert the strategy to a two-stent bifurcation culotte procedure [24]. We crossed the LCx with a Pilot 50 wire (Abbott Vascular) and opened the struts of LMS to Cx with a semi-compliant balloon Sprinter Legend 2.0 × 12 mm (Medtronic) (Fig. 2j). A 3.5 × 12 mm Promus Premier was implanted from LMS to Cx (Fig. 2k) and then potted the LMS with a 4.5 × 6mm NC Quantum Apex (Fig. 2l, m). A final kissing balloon inflation was performed with a 3.5 × 12 mm NC balloon in the LAD and 3.25 × 12 mm NC balloon in Cx (Fig. 2n and o). A good angiographic result was obtained in LMS, LAD and Cx (Fig. 2p-s) with a post-PCI IVUS in distal LMS demonstrating a stent area of 9.3 mm2 [25]. He was discharged 1 day later in a stable condition. Dual antiplatelet therapy was recommended for at least 12 months. Clinical follow-up at 6 months shows that the patient remains free of angina, with no further rehospitalizations or major adverse cardiovascular events.
| Discussion | ▴Top |
The RA is now the default route for vascular access in PCI because of its low rates of bleeding complications and the potential for early mobilization [26]. HSRA enables treatment of heavily calcified atheroma, facilitating drug-eluting stent implantation and expansion [27]. Adequate lesion preparation is paramount especially in LMS-calcified disease [28]. However, the size of the RA limits the maximum dimensions of potential GCs to 6F in most patients [17]. It is now possible to push the limits of transradial complex PCI such that 7F GCs can be used through a Slender approach [18]. The 7F Glidesheath slender (Terumo, Tokyo, Japan) is a new dedicated radial sheath with a thinner wall and hydrophilic coating. It combines an inner diameter compatible with any 7F GC and an outer diameter smaller than current 7F sheaths [29]. Nevertheless, not all RAs will allow such interventions: some cannot be cannulated using a sheath-based access and others will occlude thereafter [19]. The restriction to a 6F GC system in those patients limits the maximum size of rota burr to 1.75 mm, which can usually be delivered but will not allow sufficient contrast injection for CA. The sheathless approach overcomes all these limitations.
The 7.5F SGC Eaucath has no introducer sheath and its outer diameter is 2.49 mm smaller than the 2.70 mm of the 6F introducer sheath. It provides an inner lumen of 2.06 mm and enables the performance of HSRA with burrs measuring 2 mm or less, as described in our case. In contrast to standard GCs that have a single layer of metallic braiding, the wall of this catheter is thicker, as it has an additional layer of braiding, which provides optimal torqueability and flexibility, and an outer hydrophilic coating present along the entire length of the GC, which facilitates its smooth passage and reduces radial pain and spasm during catheter manipulation [30].
There is limited experience in performing complex LMS PCI involving HSRA via the radial approach using the 7.5F Eaucath SGC. We previously described the feasibility and safety of performing complex HRSA transradialy with the use of this catheter [16]. Twelve percent of those patients in the transradial group had significant LMS calcified disease and there was 100% procedural success [16]. Garcia-Blas et al [31] reported on their experience of transradial LMS PCI with this catheter 25 patients with HSRA with excellent results. De Maria et al [15] analyzed the immediate and long-term outcomes of transradial PCI to unprotected LMS bifurcation in a two-center retrospective registry. No significant differences were observed between the TR (244 patients) and TF (221 patients) groups in terms of 1-year mortality (10.7% versus 9.8%; P=0.79) and MACCE (18.2% versus 15.2%; P=0.44). TR patients, as compared with TF, had significantly fewer access-site complications (2.0% versus 6.3%; P=0.02), resulting in a significant reduction of net adverse clinical event rate (6.9% versus 15.7%; P=0.01). In 20% of the TR group, a 7.5F SGC was used.
In our everyday clinical practice for LMS percutaneous revascularization we try to use the TR approach with the use of this 7.5F SGC. This allows all PCI options open and being able to deliver several adjunctive devices if needed and adopt different PCI bifurcation strategies. In the presence of at least moderate calcification, a low threshold for a planned HSRA strategy to achieve adequate lesion preparation is desirable [32]. Pre- and post-stenting IVUS use is paramount as we demonstrated in our case for the following reasons: 1) qualitative assessment of plaque composition at the site of the LMS disease; and in case of calcific disease, as in our case, for the identification of the depth of the calcific component; 2) better understanding of the exact distribution of plaque burden at the LMS bifurcation which is important to decide whether a provisional stenting strategy can be applied to treat LMS disease or a two-stent strategy should be considered upfront. In our case, a limited plaque burden in the LCx ostium enabled us to start with a provisional initial strategy; 3) providing information about true vessel dimensions in order to facilitate stent sizing; and finally; 4) stent optimization as stent under-expansion is the main predictor of stent failure and, IVUS-assisted LMS PCI can be associated with a lower rate of target lesion revascularization and stent thrombosis [33]. In our case, a LMS-stented area of 9.3 mm2 conformed to the proposed Kang criteria for minimum stented area post-PCI in LMS [25].
Conclusions
LMS PCI is worthy of special considerations as compared to the treatment of coronary stenosis elsewhere in the coronary tree, with frequent involvement of the bifurcation, and often featured by a higher rate of calcific component, making LMS lesions tougher with the consequent need for appropriate and careful lesion preparation with HRSA as needed in our case. Use of IVUS is mandatory during most LMS interventions and supported by a IIaB indication in the European myocardial revascularization guidelines. Transradial percutaneous LMS revascularization can be performed safely with the use of the 7.5F Eaucath SGC when other options are not available.
Conflict of Interest
None.
| References | ▴Top |
- Kassimis G, de Maria GL, Patel N, Raina T, Scott P, Kharbanda RK, Banning AP. Assessing the left main stem in the cardiac catheterization laboratory. What is "significant"? Function, imaging or both? Cardiovasc Revasc Med. 2018;19(1 Pt A):51-56.
doi pubmed - Patel N, De Maria GL, Kassimis G, Rahimi K, Bennett D, Ludman P, Banning AP. Outcomes after emergency percutaneous coronary intervention in patients with unprotected left main stem occlusion: the BCIS national audit of percutaneous coronary intervention 6-year experience. JACC Cardiovasc Interv. 2014;7(9):969-980.
doi pubmed - Shah PJ, Durairaj M, Gordon I, Fuller J, Rosalion A, Seevanayagam S, Tatoulis J, et al. Factors affecting patency of internal thoracic artery graft: clinical and angiographic study in 1434 symptomatic patients operated between 1982 and 2002. Eur J Cardiothorac Surg. 2004;26(1):118-124.
doi pubmed - Boudriot E, Thiele H, Walther T, Liebetrau C, Boeckstegers P, Pohl T, Reichart B, et al. Randomized comparison of percutaneous coronary intervention with sirolimus-eluting stents versus coronary artery bypass grafting in unprotected left main stem stenosis. J Am Coll Cardiol. 2011;57(5):538-545.
doi pubmed - Buszman PE, Buszman PP, Kiesz RS, Bochenek A, Trela B, Konkolewska M, Wallace-Bradley D, et al. Early and long-term results of unprotected left main coronary artery stenting: the LE MANS (Left Main Coronary Artery Stenting) registry. J Am Coll Cardiol. 2009;54(16):1500-1511.
doi pubmed - Ahn JM, Roh JH, Kim YH, Park DW, Yun SC, Lee PH, Chang M, et al. Randomized Trial of Stents Versus Bypass Surgery for Left Main Coronary Artery Disease: 5-Year Outcomes of the PRECOMBAT Study. J Am Coll Cardiol. 2015;65(20):2198-2206.
doi pubmed - Morice MC, Serruys PW, Kappetein AP, Feldman TE, Stahle E, Colombo A, Mack MJ, et al. Outcomes in patients with de novo left main disease treated with either percutaneous coronary intervention using paclitaxel-eluting stents or coronary artery bypass graft treatment in the Synergy Between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) trial. Circulation. 2010;121(24):2645-2653.
doi pubmed - Authors/Task Force members, Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35(37):2541-2619.
doi pubmed - Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation. 2011;124(23):e574-651.
doi pubmed - Stone GW, Sabik JF, Serruys PW, Simonton CA, Genereux P, Puskas J, Kandzari DE, et al. Everolimus-eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med. 2016;375(23):2223-2235.
doi pubmed - Makikallio T, Holm NR, Lindsay M, Spence MS, Erglis A, Menown IB, Trovik T, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet. 2016;388(10061):2743-2752.
doi - Ancona M, Chieffo A. Percutaneous treatment of left main disease: Still learning about the optimal PCI strategy. Cardiovasc Revasc Med. 2016;17(8):494-496.
doi pubmed - Prasad SB, Malaiapan Y, Ahmar W, Meredith IT. Transradial left main stem rotational artherectomy and stenting: case report and literature review. Cardiovasc Revasc Med. 2009;10(2):136-139.
doi pubmed - Sulimov DS, Abdel-Wahab M, Toelg R, Kassner G, Geist V, Richardt G. High-speed rotational atherectomy of the left main coronary artery: a single-center experience in 50 high-risk patients. Cardiovasc Revasc Med. 2015;16(5):284-289.
doi pubmed - De Maria GL, Burzotta F, Trani C, Kassimis G, Pirozzolo G, Patel N, Dato I, et al. Trends and Outcomes of Radial Approach in Left-Main Bifurcation Percutaneous Coronary Intervention in the Drug-Eluting Stent Era: A Two-Center Registry. J Invasive Cardiol. 2015;27(7):E125-136.
pubmed - Kassimis G, Patel N, Kharbanda RK, Channon KM, Banning AP. High-speed rotational atherectomy using the radial artery approach and a sheathless guide: a single-centre comparison with the "conventional" femoral approach. EuroIntervention. 2014;10(6):694-699.
doi pubmed - Saito S, Ikei H, Hosokawa G, Tanaka S. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc Interv. 1999;46(2):173-178.
doi - Sanon S, Gulati R. Slender Approach and Sheathless Techniques. Interv Cardiol Clin. 2015;4(2):161-166.
doi - Levin D, Adawi S, Halon DA, Shiran A, Asmer I, Rubinshtein R, Jaffe R. Long-Term Radial Artery Patency Following Transradial Coronary Catheterization via a 7-Fr Sheath. Isr Med Assoc J. 2016;18(5):290-293.
pubmed - Youn YJ, Yoon J, Han SW, Lee JW, Sung JK, Ahn SG, Kim JY, et al. Feasibility of transradial coronary intervention using a sheathless guiding catheter in patients with small radial artery. Korean Circ J. 2011;41(3):143-148.
doi pubmed - de la Torre Hernandez JM, Hernandez Hernandez F, Alfonso F, Rumoroso JR, Lopez-Palop R, Sadaba M, Carrillo P, et al. Prospective application of pre-defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesions results from the multicenter LITRO study. J Am Coll Cardiol. 2011;58(4):351-358.
doi pubmed - Kang SJ, Ahn JM, Kim WJ, Lee JY, Park DW, Lee SW, Kim YH, et al. Functional and morphological assessment of side branch after left main coronary artery bifurcation stenting with cross-over technique. Catheter Cardiovasc Interv. 2014;83(4):545-552.
doi pubmed - Burzotta F, Lassen JF, Banning AP, Lefevre T, Hildick-Smith D, Chieffo A, Darremont O, et al. Percutaneous coronary intervention in left main coronary artery disease: the 13th consensus document from the European Bifurcation Club. EuroIntervention. 2018;14(1):112-120.
doi pubmed - Ahn JM, Lee JY, Kang SJ, Kim YH, Song HG, Oh JH, Park JS, et al. Functional assessment of jailed side branches in coronary bifurcation lesions using fractional flow reserve. JACC Cardiovasc Interv. 2012;5(2):155-161.
doi pubmed - Kang SJ, Ahn JM, Song H, Kim WJ, Lee JY, Park DW, Yun SC, et al. Comprehensive intravascular ultrasound assessment of stent area and its impact on restenosis and adverse cardiac events in 403 patients with unprotected left main disease. Circ Cardiovasc Interv. 2011;4(6):562-569.
doi pubmed - Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157(1):132-140.
doi pubmed - Abdel-Wahab M, Richardt G, Joachim Buttner H, Toelg R, Geist V, Meinertz T, Schofer J, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2013;6(1):10-19.
doi pubmed - De Maria GL, Patel N, Banning AP. Obstructive left main stem coronary disease: is it time to recommend coronary stenting? Heart. 2018;104(7):614-620.
pubmed - Aminian A, Iglesias JF, Van Mieghem C, Zuffi A, Ferrara A, Manih R, Dolatabadi D, et al. First prospective multicenter experience with the 7 French Glidesheath slender for complex transradial coronary interventions. Catheter Cardiovasc Interv. 2017;89(6):1014-1020.
doi pubmed - Rathore S, Stables RH, Pauriah M, Hakeem A, Mills JD, Palmer ND, Perry RA, et al. Impact of length and hydrophilic coating of the introducer sheath on radial artery spasm during transradial coronary intervention: a randomized study. JACC Cardiovasc Interv. 2010;3(5):475-483.
doi pubmed - Garcia-Blas S, Nunez J, Carrillo P, Cordero A, Minana G, Frutos A, Valero E, et al. Usefulness of sheathless guide catheter for the percutaneous coronary intervention of left main disease by radial approach. Int J Cardiol. 2016;211:49-52.
doi pubmed - Barbato E, Carrie D, Dardas P, Fajadet J, Gaul G, Haude M, Khashaba A, et al. European expert consensus on rotational atherectomy. EuroIntervention. 2015;11(1):30-36.
doi pubmed - Nerlekar N, Cheshire CJ, Verma KP, Ihdayhid AR, McCormick LM, Cameron JD, Bennett MR, et al. Intravascular ultrasound guidance improves clinical outcomes during implantation of both first- and second-generation drug-eluting stents: a meta-analysis. EuroIntervention. 2017;12(13):1632-1642.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


