| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website http://www.cardiologyres.org |
Original Article
Volume 9, Number 6, December 2018, pages 364-369
Pharmacokinetic Study of Sirolimus-Eluting BioResorbable Vascular Scaffold System for Treatment of De Novo Native Coronary Lesions: A Sub-Study of MeRes-1 Trial
Praveen Chandraa, d, Ajaykumar U. Mahajanb, d, Vipin D. Bulanic, Ashok S. Thakkarc
aMedanta, Gurgaon, India
bLokmanya Tilak Municipal Medical College and General Hospital, Mumbai, India
cMeril Life Sciences Pvt. Ltd., Vapi, Gujarat, 396191, India
dCorresponding Author: Praveen Chandra, Interventional Cardiology, Medanta, The Medcity, Sector-38, Gurgaon, Haryana 122 001, India; Ajaykumar U. Mahajan, Department of Cardiology, L.T.M. Medical College and General Hospital, Sion, Mumbai, Maharashtra 400022, India
Manuscript submitted October 26, 2018, accepted November 12, 2018
Short title: MeRes100™ BRS Pharmacokinetic
doi: https://doi.org/10.14740/cr799
| Abstract | ▴Top |
Background: MeRes100™ (Meril Life Sciences Pvt. Ltd., Vapi, India) is a novel sirolimus-eluting bioresorbable vascular scaffold (BRS). The purpose of this sub-study of MeRes-1 trial is to evaluate the systemic release of sirolimus from MeRes100 BRS implanted for the treatment of de novo native coronary artery lesions.
Methods: The MeRes-1 is a prospective, multicenter, first-in-human trial of sirolimus-eluting MeRes100 BRS. The pharmacokinetic sub-study was conducted at two Indian sites in 10 patients who were implanted with the MeRes100 BRS loaded with sirolimus at a dose of 1.25 µg/mm2. Venous blood samples were collected at pre-dose and 12-time points after implantation of the scaffold. Sirolimus concentration was successively analyzed using ultra-performance liquid chromatography-electrospray ionization tandem mass spectrometry method.
Results: A total of 12 scaffolds were implanted in 10 patients. Non-compartmental analysis demonstrated time to reach peak concentration of sirolimus between 0.5 h to 3 h after scaffold implantation. The peak concentration (Cmax) was deduced to be 7.47 ± 2.61 ng/mL, AUC was 436.45 ± 171.24 h·ng/mL, and the t½ was observed at 98.59 ± 33.58 h. The clearance was 0.66 ± 0.16 L/h and lower limit of quantification was detectable at 14.1 days.
Conclusions: The MeRes-1 pharmacokinetic sub-study confirmed that MeRes100 BRS is safe and tolerable at limited systemic exposure of sirolimus.
Keywords: Bioresorbable vascular scaffold; Pharmacokinetics; Sirolimus
| Introduction | ▴Top |
Bioresorbable vascular scaffold (BRS) is an innovative technique allowing to maintain endothelial dysfunction and avoid creation of a permanent stent cage vessel segment without inflammation over the course of the patient’s life [1]. In addition, bioresorbable vascular scaffolds provide adequate mechanical support required to prevent vessel recoil till 12 months, release the anti-proliferative drug for prevention of restenosis, and leaving nothing behind over the long-term [2, 3]. Sirolimus is a lipophilic macrocyclic lactone with a potent immunosuppressive activity. It prolongs allogeneic transplant survival in humans and animal models [4, 5]. The reduction of hyperplastic vascular smooth muscle cell growth and inflammatory inhibition within the neointima of the treated coronary artery are achieved by local release of therapeutic levels of sirolimus drug [6].
The newly designed MeRes100 (Meril Life Sciences Pvt. Ltd., Vapi, India) sirolimus-eluting BRS is composed of biocompatible and biodegradable polymer poly-L-lactic acid (PLLA). The MeRes100 BRS was developed to reduce in-stent restenosis and late stent thrombosis in coronary artery lesions. These adversities substantially reduce the performance of bare-metal stents and drug-eluting stents (DES). Furthermore, the life-long presence of the metallic prosthesis prevents restoration of vasomotion, restricts quality lesion imaging and interferes with repeat surgical or percutaneous treatment [7-9]. Drug release kinetics of sirolimus plays an important role in establishing safety and efficacy of the BRS. In order to prevent in-stent restenosis and stent thrombosis, a locally effective drug concentration is required which can prevent cell proliferation in the neointima of the coronary artery. On the other hand, low levels of systemic sirolimus concentrations are desirable to avoid complications related to the immunosuppressive property of the drug.
The pharmacokinetics (PK) of sirolimus released from DES has been studied earlier [10, 11]. However, there is a paucity of data which explains PK of sirolimus releasing from BRS after implantation. Previously, we have demonstrated clinical outcomes and vascular responses by multiple imaging assessments of MeRes100 at 1-year follow-up [12]. This is the first report describing the drug release kinetics of sirolimus from the MeRes100 BRS in patients with de novo coronary artery lesion.
| Materials and Methods | ▴Top |
Study design
This study was a prospective, single-arm, open-label pilot study conducted at two Indian sites in which patients received either one or two MeRes100 BRS. Out of the 10 patients enrolled in the study, eight patients were implanted with single BRS and the rest two patients were implanted with two BRS. A total of 12 MeRes100 BRS loaded with 1.25 µg/mm2 sirolimus were used in patients with de novo coronary artery lesion.
The present study was performed in compliance with ICH-GCP guidelines, the Declaration of Helsinki, ISO 14155 and all other local regulatory requirements. This study was conducted with the approval of local ethics committee. Informed written consent was obtained from all study participants. The MeRes-1 trial was registered at the Clinical Trials Registry, India. CTRI number: CTRI/2015/04/005706.
Inclusion criteria
All included patients were aged between 18 to 65 years. Major inclusion criteria included stable, unstable or silent ischemic heart disease, the patient who presented had stenosis of > 50% and < 100% with a thrombolysis in myocardial infarction (TIMI) flow grade of ≥ 1 was eligible for the trial. Patients with up to two de novo coronary lesions (maximum one per target vessel) with a reference vessel diameter of 2.75 - 3.50 mm and lesion length ≤ 20 mm were included.
Exclusion criteria
Any patient with a known allergy to PLLA and/or poly (D, L-lactide) (PDLLA), sirolimus, and aspirin with P2Y12 inhibitors were excluded from the study. Other exclusion criteria were diagnosis of acute myocardial infarction within preceding 7 days of the procedure, history of previous revascularization with coronary artery bypass graft (CABG) or percutaneous coronary intervention, presence of vascular aneurysms, cardiac arrhythmias, left ventricular ejection fraction (LVEF) < 30%, and cardiac tamponade. Pregnant women and breastfeeding mothers, women who were planning to conceive within 1 year of BRS implantations, or the patients awaiting any form of organ transplantation were excluded from the study. The main angiographic exclusion criteria were: lesion located in left main coronary artery or aorto-ostial location (within 3 mm); lesion that involves a bifurcation with a side branch ≥ 2 mm in diameter and ostial lesion > 40% stenosis; total occlusion TIMI flow 0); evidence of severe tortuosity and angulation of target vessel.
Study device
MeRes100 is a balloon-expandable BRS with a polymeric backbone made up of biodegradable PLLA. The PLLA backbone carries a thin coating of 1:1 formulation of PDLLA polymer and anti-proliferative drug sirolimus. The PDLLA controls release of the drug and forms an amorphous reservoir for sirolimus with a concentration of 1.25 µg/mm2. Both PLLA and PDLLA are biodegradable polymers which undergo hydrolytic degradation in the body resulting in mass loss and bulk erosion. The hydrolysis of the ester bonds in the polymers generates lactic acid which enters into Kreb’s cycle and converted to CO2 and H2O which is eliminated out of the body. This allows for complete disappearance of the scaffold from the treatment site within 36 months of implantation. MeRes100 BRS is a thin strut (100 µm) BRS which is easily deployable since it has three radiopaque markers at each end which allow convenient viewing of the scaffold in two orthogonal views during its deployment. In present study, MeRes100 BRS with diameters of 2.75, 3.00, and 3.50 mm, and lengths of 19 and 24 mm were implanted.
Study procedure
The target lesions were treated according to standard guideline for percutaneous transluminal coronary angioplasty procedure [13]. Pre-dilatation was maintained up to its nominal pressure while post-dilatation was carried out at ≥ 18 atm pressure with non-compliant balloon (not exceeding 0.5 mm nominal diameter of implanted stent) to achieve residual diameter stenosis of ≤ 10%. Loading dose of aspirin and clopidogrel were given prior to the index procedure. Dual antiplatelet therapy with aspirin (75 - 150 mg/day) and clopidogrel (75 mg/day) or prasugrel (10 mg/day) or ticagrelor (90 mg/day) was maintained for minimum duration of 1 year. Further administration of aspirin alone was left to the operator’s discretion.
Blood sampling
A total of 13 blood samples (each 6 mL) from each patient were collected including pre-dose before (-10 min) and after scaffold implantation of 10 min (± 2min), 30 min (± 2min), 1 h (± 5min), 3 h (± 5min), 6 h (± 5min), 12 h (± 10min), 24 h (± 10min), 7 days (± 1 day), 14 days (± 1 day), 30 days (± 2 days), 60 days (± 2 days) and 90 days (± 2 days). All the blood samples were anticoagulated with potassium EDTA and stored at -78 ± 8 °C until analysis. Venous blood samples were analyzed at Veeda Clinical Research Pvt. Ltd., Ahmedabad, India.
Laboratory analysis
Whole blood concentration of sirolimus was analyzed using ultra-performance liquid chromatography-electrospray ionization tandem mass spectrometry method with lower limit of quantification (LLOQ) of 0.1 ng/mL and upper limit of quantification (ULOQ) of 50 ng/mL.
Statistical analysis
The PK analysis of sirolimus was performed using non-compartmental model by using WinNonlin® Enterprise Software Version 5.3 (Pharsight Corporation, USA). The analysed PK parameters were peak concentration (Cmax, ng/mL), time to reach Cmax (Tmax), area under curve [14], half-life (t1/2), clearance (CL) and elimination rate constant (kel). Data were expressed as mean ± standard deviation (SD).
| Results | ▴Top |
Baseline demographics characteristics
Ten patients were included in the study and follow-up was obtained from all patients. The mean age of the patients was 49.07 ± 8.95 years and seven (70%) patients were male. A total of 12 BRS were deployed in the study patients where seven patients received single BRS and out of three patients having double-vessel disease with two patients received two BRS and one patient deployed with single BRS. Baseline characteristics for risk factors shows that two (20%) patients were smokers; four (40%) had diabetes; three (30%) had hypertension; and three (30%) had a history of myocardial infarction (Table 1). The mean LVEF for the patients was 59.5±3.02%.
 Click to view | Table 1. Baseline Demographics and Clinical Presentation |
Dose range
The patients were implanted with MeRes100 BRS of variable length and diameter. The total loading sirolimus dose was calculated based on the drug coating of sirolimus (1.25 µg /mm2). All patients received loading dose of sirolimus ranged from 238 µg to 467 µg. Detailed specifications of MeRes100 BRS are outlined in Table 2.
 Click to view | Table 2. Corresponding Loading Dose of Sirolimus Received by Each Patient |
Pharmacokinetic results
The PK parameter outcomes for individual patients received one or two stents and for the entire patient population are summarized in Table 3. A total of 10 patients were enrolled in the study and individual Cmax ranged from 3.88 ng/mL to 10.71 ng/mL was achieved between 0.5 - 3.0 h. The mean peak blood sirolimus concentration of patients receiving two BRS was higher than the patients receiving single BRS (9.72 ± 1.41 vs. 6.91 ± 2.58 ng/mL). Also, all the AUC parameters which represent the systemic exposure of sirolimus were higher in two BRS implanted patients than single BRS deployed patients. In addition, the parameters representing the release of sirolimus from the scaffold-like Tmax (1.75 ± 1.77 vs. 1.44 ± 0.98 h), t1/2 (99.96 ± 5.83 vs. 98.25 ± 38.00 h), CL (0.67 ± 0.18 vs. 0.63 ± 0.06 L/h) were almost similar for patients receiving two BRS and single BRS respectively.
 Click to view | Table 3. Individual and Mean Pharmacokinetic Parameters of Sirolimus |
The blood sirolimus concentrations declined rapidly within 2 h of BRS implantation followed by biphasic elimination. The sirolimus blood concentrations remained above LLOQ at 340 h (14.1 days) after implantation of BRS. The mean PK outcomes for all patients was estimated 7.47 ± 2.61 ng/mL for Cmax; 1.50 ±1.05 h for Tmax; 98.59 ± 33.58 h for t1/2; 0.66 ± 0.16 L/h for CL; 436.46 ± 171.25 h·ng/mL for AUC0-t; and 469.33 ± 184.67 h·ng/mL for AUC0-∞. Drug concentration versus time profile of 10 patients for 24 h and over 90 days after implantation of sirolimus-eluting MeRes100 BRS is represented in Figure 1.
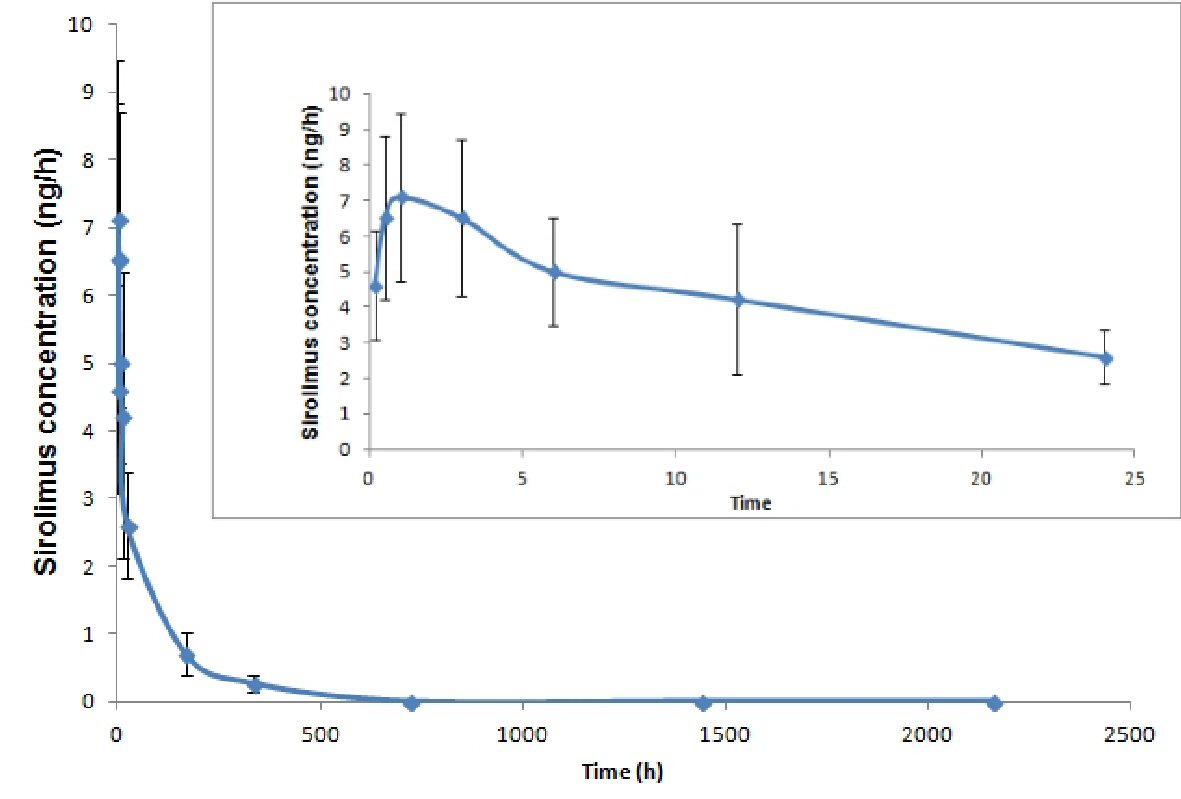 Click for large image | Figure 1. Pharmacokinetic profiles (concentration versus time profile) of sirolimus over 90 days after implantation of sirolimus-eluting MeRes100 BRS. |
| Discussion | ▴Top |
The MeRes-1 trial is the first study to assess systemic exposure of sirolimus eluted from a BRS implanted to treat de novo native coronary artery lesions. The immunosuppressive property of sirolimus improves allograft survival and prevents graft rejection in renal, pancreatic islet cell, liver and heart transplantation [15]. Moreover, prophylaxis treatment with sirolimus is used to prevent graft-versus-host-disease [16]. Clinical trials demonstrated that systemic sirolimus prevents graft rejection at blood concentration of 8 - 17 ng/mL. In this observation, systemic sirolimus represents various side-effects, including headaches, cytopenia, polyarthralgia, stomatitis, epistaxis, diarrhea, skin disorders and dyslipidemia [5, 17]. The mean Cmax of sirolimus eluted from MeRes100 is 7.47 ± 2.61 ng/mL and peak blood concentrations declined rapidly within 2 h after BRS implantation. This implies that MeRes100 BRS containing sirolimus as an anti-restenotic drug is safe to use and will not lead to any immunosuppression-related adverse events.
When local drug concentrations in human target tissues are required, animal model pharmacokinetics is used to predict the drug kinetics in humans. Studies have used animal models to predict vascular response in humans after implantation of DES [18]. Effectiveness of direct intramural delivery of sirolimus to prevent vascular remodeling after balloon angioplasty of coronary lesions was studied in rabbit iliac models. It was found that IC50 of 5.8 nM (5.3 ng/mL) is required for significant reduction of platelet derived growth factor (PDGF)-induced proliferation [19]. The mean peak systemic sirolimus concentrations observed in the present study was 7.47 ± 2.61 ng/mL suggesting adequate local drug concentrations. Similarly, one recently published 2-year results of the MeRes100 BRS showed a similar pharmacokinetic profile in pig models, with the peak concentration of sirolimus in blood occurred at 1 - 4 h and the mean peak systemic concentration was 7.38 ± 0.42 ng/mL after scaffold deployment [20].
The sufficient localized drug delivery of sirolimus from the MeRes100 BRS may help in reducing in-scaffold late lumen loss and binary restenosis [12]. In present study, patients received loading dose of sirolimus between 238 and 467 µg elucidating Cmax of sirolimus between 0.5 and 3 h after scaffold implantation. This result is similar to prior PK study of Absorb™ (Abbott Vascular, Santa Clara, CA, USA) BRS with loading dose of everolimus between 181 and 443 µg which demonstrated rapid increase in systemic everolimus levels after scaffold deployment, reaching peak concentrations within 2.5 h [21].
In a particular clinical setting, estimation of drug clearance is an important parameter to identify its elimination outside the body. The mean clearance of sirolimus eluted from MeRes100 BRS was 0.66 ± 0.16 L/h. This value was lower than the mean clearance (2.16 L/h) reported for healthy subjects who received oral sirolimus [22]. However, the clearance value was comparable with other sirolimus-eluting metallic stents such as Supralimus-Core® (0.83 ± 0.21 L/h) and Bx-Velocity® (1.46 ± 0.45 L/h) [10, 11]. Continuous drug availability through prolonged drug release from the DES and BRS is likely to contribute to prolonged clearance time.
Previous study demonstrated that t1/2 of everolimus was 87.4 ± 53.0 h at dose > 150 µg in XIENCE V implanted patients [14]. Similarly, for MeRes100 BRS, t1/2 of sirolimus was 98.59 ± 33.58 h at dose of 238 to 467 µg. The slightly higher t1/2 values in the present study are attributed to prolonged drug release from the PDLLA reservoir of BRS as well as partitioned into the lipid-rich arterial wall, thereby limiting systemic drug delivery. In the present study, absence of sirolimus toxicity confirms that adequate systemic exposure of the drug elicits its good tolerability and safety profile.
Few limitations of this study includes: 1) Single-arm, pilot study with a small number of patients; 2) This was a non-randomised study without comparison groups. Despite these limitations, this analysis supports the information on the PK profile and safety of sirolimus-eluting MeRes100 BRS in patients with de novo native coronary artery lesions.
Conclusions
In summary, steady drug availability via extended drug release also most likely contributes to the prolonged clearance time. The study confirms limited systemic exposure of sirolimus from the MeRes100 BRS suggesting safe and tolerable in terms of systemic toxicity.
Funding
Meril Life Sciences Pvt. Ltd. is the sponsor of the MeRes-1 trial.
Conflict of Interest
Dr. Ashok Thakkar and Dr. Vipin Bulani are full-time employee of Meril Life Sciences Pvt. Ltd., India. The other authors have no potential conflict of interest to declare.
| References | ▴Top |
- Gao R, Yang Y, Han Y, Huo Y, Chen J, Yu B, Su X, et al. Bioresorbable vascular scaffolds versus metallic stents in patients with coronary artery disease: ABSORB China Trial. J Am Coll Cardiol. 2015;66(21):2298-2309.
doi pubmed - Alexy RD, Levi DS. Materials and manufacturing technologies available for production of a pediatric bioabsorbable stent. Biomed Res Int. 2013;2013:137985.
- Stone GW. Bioresorbable Vascular Scaffolds: More Different Than Alike? JACC Cardiovasc Interv. 2016;9(6):575-577.
doi pubmed - Stepkowski SM. Preclinical results of sirolimus treatment in transplant models. Transplant Proc. 2003;35(3 Suppl):219S-226S.
doi - Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet. 2000;356(9225):194-202.
doi - Mahalati K, Kahan BD. Clinical pharmacokinetics of sirolimus. Clin Pharmacokinet. 2001;40(8):573-585.
doi pubmed - Serruys PW, Ormiston JA, Onuma Y, Regar E, Gonzalo N, Garcia-Garcia HM, Nieman K, et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373(9667):897-910.
doi - Serruys PW, Onuma Y, Ormiston JA, de Bruyne B, Regar E, Dudek D, Thuesen L, et al. Evaluation of the second generation of a bioresorbable everolimus drug-eluting vascular scaffold for treatment of de novo coronary artery stenosis: six-month clinical and imaging outcomes. Circulation. 2010;122(22):2301-2312.
doi pubmed - Diletti R, Serruys PW, Farooq V, Sudhir K, Dorange C, Miquel-Hebert K, Veldhof S, et al. ABSORB II randomized controlled trial: a clinical evaluation to compare the safety, efficacy, and performance of the Absorb everolimus-eluting bioresorbable vascular scaffold system against the XIENCE everolimus-eluting coronary stent system in the treatment of subjects with ischemic heart disease caused by de novo native coronary artery lesions: rationale and study design. Am Heart J. 2012;164(5):654-663.
doi pubmed - Thakkar AS, Abhyankar AD, Dani SI, Banker DN, Singh PI, Parmar SA, Mehta AA. Systemic exposure of sirolimus after coronary stent implantation in patients with de novo coronary lesions: Supralimus-Core(R) pharmacokinetic study. Indian Heart J. 2012;64(3):273-279.
doi - Vetrovec GW, Rizik D, Williard C, Snead D, Piotrovski V, Kopia G. Sirolimus PK trial: a pharmacokinetic study of the sirolimus-eluting Bx velocity stent in patients with de novo coronary lesions. Catheter Cardiovasc Interv. 2006;67(1):32-37.
doi pubmed - Seth A, Onuma Y, Costa R, Chandra P, Bahl VK, Manjunath CN, Mahajan AU, et al. First-in-human evaluation of a novel poly-L-lactide based sirolimus-eluting bioresorbable vascular scaffold for the treatment of de novo native coronary artery lesions: MeRes-1 trial. EuroIntervention. 2017;13(4):415-423.
doi pubmed - Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124(23):e574-651.
pubmed - Wang Q, Pierson W, Sood P, Bol C, Cannon L, Gordon P, Saucedo J, et al. Pharmacokinetic sub-study in the SPIRIT III Randomized and Controlled Trial of XIENCE V everolimus eluting coronary stent system. J Interv Cardiol. 2010;23(1):26-32.
doi pubmed - Stenton SB, Partovi N, Ensom MH. Sirolimus: the evidence for clinical pharmacokinetic monitoring. Clin Pharmacokinet. 2005;44(8):769-786.
doi pubmed - Cutler C, Antin JH. Sirolimus for GVHD prophylaxis in allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;34(6):471-476.
doi pubmed - Klugherz BD, Llanos G, Lieuallen W, Kopia GA, Papandreou G, Narayan P, Sasseen B, et al. Twenty-eight-day efficacy and phamacokinetics of the sirolimus-eluting stent. Coron Artery Dis. 2002;13(3):183-188.
doi pubmed - Virmani R, Kolodgie FD, Farb A, Lafont A. Drug eluting stents: are human and animal studies comparable? Heart. 2003;89(2):133-138.
doi pubmed - Buerke M, Guckenbiehl M, Schwertz H, Buerke U, Hilker M, Platsch H, Richert J, et al. Intramural delivery of Sirolimus prevents vascular remodeling following balloon injury. Biochim Biophys Acta. 2007;1774(1):5-15.
doi pubmed - Gasior P, Cheng Y, Xia J, Conditt GB, McGregor JC, Virmani R, Granada JF, et al. Two-year longitudinal evaluation of a second-generation thin-strut sirolimus-eluting bioresorbable coronary scaffold with hybrid cell design in porcine coronary arteries. Cardiol J. 2018.
- Rizik DG, Cannon L, Stone GW, Kennedy M, Piard-Ruster K, Staehr P, Ellis SG, et al. Systemic pharmacokinetics of everolimus eluted from the absorb bioresorbable vascular scaffold: an ABSORB III substudy. J Am Coll Cardiol. 2015;66(21):2467-2469.
doi pubmed - Zheng J, Sambol N, Zimmerman D, Zaidi A. Population pharmacokinetics (PK) of sirolimus. Clinical Pharmacology & Therapeutics. 1996;59(2):150-150.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


