| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website http://www.cardiologyres.org |
Original Article
Volume 10, Number 2, April 2019, pages 98-105
Rate Control Yields Better Clinical Outcomes Over a Median Follow-Up of 20 Months Compared to Rhythm Control Strategy in Patients With a History of Atrial Fibrillation: A Retrospective Cohort Study
Renato De Vecchisa, c, Marco Di Maiob, Silvia Sorecaa, Carmelina Arianoa
aPreventive Cardiology and Rehabilitation Unit, DSB 29 “S. Gennaro dei Poveri Hospital”, via S.Gennaro dei Poveri 25, 80136 Naples, Italy
bDepartment of Cardiology, University of Campania “Luigi Vanvitelli”, 80138 Naples, Italy
cCorresponding Author: Renato De Vecchis, Preventive Cardiology and Rehabilitation Unit, DSB 29 “S. Gennaro dei Poveri Hospital”, via S.Gennaro dei Poveri 25, 80136 Naples, Italy
Manuscript submitted December 28, 2018, accepted February 13, 2019
Short title: Comparison of Pharmacological Regimens for AF
doi: https://doi.org/10.14740/cr829
| Abstract | ▴Top |
Background: Clinical management of patients with a history of atrial fibrillation (AF) focuses on the goal of preventing AF recurrences, or, if this is impossible due to the fact that the arrhythmia has by now become permanent, it is aimed at the control of the ventricular response. In patients with AF, an important topic is the comparative evaluation in the mid/long-term of clinical outcomes arising from the various therapeutic regimens, including pharmacological approaches as well as radiofrequency catheter ablation (abl).
Methods: In the present cohort retrospective study, 175 cases of paroxysmal, persistent or long-lasting persistent AF have been grouped depending on therapeutic approach: abl-isolated or followed by chronic use of antiarrhythmics (74 cases), drug treatment for rate control strategy (60 cases), drug treatment for rhythm control strategy (41 cases). The effects respectively exerted by the three treatment modalities on the primary endpoint, namely a composite of death, disabling stroke, severe bleeding and cardiac arrest , have been compared through a median follow-up of 20 months (interquartile range = 18 - 24 months) using the Cox proportional-hazards regression analysis.
Results: As documented by the Cox model, an increased risk of the primary composite endpoint was associated with the rhythm control strategy, as well as with the AF recurrences during the follow-up (for the former, hazard ratio (HR): 3.3159, 95% CI: 1.5415 to 7.1329, P = 0.0023; for the latter, HR: 1.0448, 95% CI: 1.0020 to 1.0895, P = 0.0410). Even hypertension was associated with an increased risk (HR: 1.1040; 95% CI: 1.0112 to 1.9662; P = 0.0477). On the contrary, a rate control strategy predicted a decreased risk of experiencing the primary endpoint (HR: 0.0711; 95% CI: 0.0135 to 0.3738; P = 0.0019) while abl did not exert a statistically significant effect on the same outcome.
Conclusions: AF abl decreases the arrhythmic episodes but does not provide a statistically significant protection against the composite of death, disabling stroke, major bleeding and cardiac arrest after a 20-month follow-up. Moreover, in patients with a history of AF, rate control compared to rhythm control strategy provides better clinical outcomes over a mid-term follow-up.
Keywords: Atrial fibrillation; Rate control strategy; Rhythm control strategy; Clinical outcomes
| Introduction | ▴Top |
Transcatheter ablation is the most advanced approach to the treatment of paroxysmal, persistent and long-lasting persistent atrial fibrillation (AF) [1]. It consists of the electro-anatomical isolation of the pulmonary venous ostia within the left atrium after puncturing the interatrial septum and introducing the operative catheter into the left atrium. The technique in question was set up for the first time by Haissaguerre et al [2] and refined later by Pappone et al [3]. The creation of linear lesions with the radiofrequency catheter at the level of these arrhythmogenic foci is then complemented in selected cases by the induction of additional lesions of variable extent of the subendocardial layers of the atrial myocardium. Unlike the very targeted approaches used for the selective ablation of the accessory idionodal [4] or atrio-ventricular pathways [5], the extensively destructive character of the AF ablation technique has always prompted, from its first appearance, hesitations and perplexities. They are essentially about the concept that with atrial ablation one artificially creates areas of inhomogeneity of impulse conduction that could take the function of a trigger or of re-entry pathway, in analogy with what is observed at the level of the ventricular myocardium injured by the infarct. In fact it is known that fibrotic areas or post-infarct scars are able to act as a starting and triggering point for even severe hyperkinetic arrhythmias. Therefore, there are still some conceptual reluctances and tenacious objections regarding the option of treating AF by creating radiofrequency-related atrial cicatrices which might be potentially arrhythmogenic in themselves [6].
Aims of the study
In the present study, banning the conceptual diatribes and operational disputes, we have rather considered the widespread diffusion of radiofrequency ablation techniques to make a retrospective comparison between ablated patients and patients subjected to pharmacological antiarrhythmic therapy within a population consisting of patients with recent occurrence of AF, paroxysmal, persistent or long-lasting persistent AF. We have considered a composite primary endpoint, consisting of death, disabling stroke, severe bleeding and cardiac arrest, over a mid-term follow-up. In addition, as a secondary endpoint, the number of AF relapses comparatively evaluated in the ablated patients versus those pharmacologically treated has been taken into account.
| Materials and Methods | ▴Top |
The study was designed as a retrospective cohort study. The main task of our team was the collection of data derived from a median follow-up of 20 months, concerning the two above-mentioned endpoints. We have retrospectively collected the data because at that time (2015 - 2017) this was the only available modality for the execution of such a study (in particular, it was not possible for us to implement the procedures of AF ablation).
It is important to note that the procedures described in the study were carried out by medical-health professionals and technicians in the field of electrophysiology and interventional arrhythmology who do not coincide with those, the article’s authors who at a later stage extracted the archived data and performed statistical comparisons.
In other words, the ablation techniques and pharmacological treatments, with the choice of drugs and the selection of the respective dosages, were performed by doctors who subsequently had no role in drafting the article or making comparative evaluations between groups and in processing and interpretation of the data.
As regards the origin of the data used for making comparisons, it is largely composite due to the fact that the pertaining database collects contributions attributable to patients belonging to the Cardiology Outpatient Units and Inpatient Divisions of the ASL Napoli 1 Centro (Naples, Italy), as well as those coming from Cardiac Arrhythmology Operational Unit of the “Pineta Grande” Clinic of Castelvolturno (CE), Italy, or those derived from the Complex Structure of Cardiac Arrhythmology and Electrophysiology of the “San Raffaele” IRCCS Hospital in Milan, Italy.
Patient selection
The patients who underwent ablation as secondary prevention for their AF were all subjected to pulmonary vein isolation (PVI) by point-by-point radiofrequency ablation.
Procedural endpoint was ipsilateral PVI [1] assessed by a circular mapping catheter and challenged by adenosine. Ablation at the cavo-tricuspid isthmus was performed in patients with documented typical atrial flutter. Patients were usually discharged the day after the ablation procedure, and oral anticoagulation was continued for at least 3 months. The blanking period was usually kept for a duration ranging from 1 to 3 months, during which it was customary practice to administer an oral antiarrhythmic maintenance therapy (drugs of classes IC or III of the classification of Vaughan Williams, in particular propafenone, flecainide and sotalol, only rarely amiodarone at congruous doses per os). Based on the documentation, both paper and electronic, it was possible to identify a dichotomy of prescriptive behaviors by the physicians charged of clinical management of the patients previously undergone AF ablation.
In fact, at cardiologist’s complete discretion, in ablated patients oral antiarrhythmic therapy (IC drugs or sotalol) could be discontinued at the end of the canonical blanking period, or, on the contrary, it could be kept according to doses established by empirical criteria, and according to completely arbitrary timing (up to 6 months after ablation, up to 12 months after ablation or indefinitely).
Importantly, the physicians prescribing drugs for rate or rhythm control strategy almost always forgot to detail in the clinical sheet the guiding criteria and/or rational motivations for the solutions to be applied to individual patients. In fact, from the thorough analysis of the clinical picture (in particular, possible comorbidities, possible coexistence of signs and symptoms of heart failure, and echocardiographic measurements of the left atrium), as well as from the chronological characterization of atrial fibrillation (if paroxysmal, persistent, or long lasting persistent), it was not consistently possible to identify a rational criterion, capable of systematically justifying the indication of the doctors to use the rate control instead of the rhythm control strategy or vice versa. In order to make the baseline clinical and echocardiographic data as homogeneous as possible, patients were not included in bulk in the study. On the contrary, we tried to collect a patient population as uniform as possible.
Patient follow-up
During the follow-up, after the ablation procedures or the initiation of anti-arrhythmic pharmacological prophylaxis according to the rate or rhythm control strategy, the patients performed clinical visits as well as instrumental checks (collection of the anamnesis, objective clinical examination, 12-lead surface ECG, 24-h Holter examination, and transthoracic echocardiogram) at intervals of 3 months or even more often, in case of justified clinical reasons (e.g., AF relapse in the meantime).
The retrospective review of the medical records that were used in the study preparation involved the years 2017 and 2016, and 2015 in part. In any case these time limits allowed the authors to collect a sufficient number of patients to be included in the statistical comparisons necessary to the retrospective study and to the article’s preparation. Patients were encouraged to register arrhythmia-related symptoms and to have an ECG taken during symptoms. In case of long-lasting arrhythmia symptoms, patients were instructed not to change drug treatment and to contact their cardiologist or their local hospital.
In any case, patients were informed about their inclusion in anonymous form in the analysis of aggregate data and they consistently consented being included in this retrospective study, after being made aware of its modalities and scientific aims.
Statistical analysis
Continuous data are reported as means (± SD) or medians (interquartile range (IQR)). D’Agostino-Pearson test was used to test for normality. Comparisons between groups were performed using the Student’s t-test or Mann-Whitney U test where appropriate. Categorical data were reported as proportions or percentages and comparisons between groups were performed using the Chi-squared test or the Fisher’s exact test where appropriate. Likewise, when necessary, Kruskal-Wallis as well as one-way ANOVA tests was carried out.
Analyses were performed according to the modified intention-to-treat principle. The primary endpoint ,i.e., the composite consisting of death, disabling stroke, severe bleeding and cardiac arrest, was considered as an outcome variable within a multivariable Cox proportional-hazards regression model comprising several exposure variables. Multivariate Cox proportional-hazards regression was also employed to assess possible predictors for AF recurrences during the follow-up (secondary endpoint). Statistical tests were two tailed, and P-value ≤ 0.05 was considered statistically significant. Analyses were conducted using Excel 2016 (version 16.0, Seattle, WA, USA) as well as MedCalc Version 18.6 (Acacialaan 22, 8400 Ostend, Belgium).
| Results | ▴Top |
In the present study, the patients, assigned to the three different modalities of prophylaxis of AF recurrences, exhibited strong similarities concerning the clinical history and basal features. In this regard, we refer to Table 1 that includes some anthropometric, clinical and echocardiographic characteristics of each of the three therapeutic arms (the patients undergone ablation, those undergone rate control strategy and those treated with rhythm control strategy).
 Click to view | Table 1. Clinical Characteristics |
Importantly, among the 194 patients originally gathered for enrollment in the retrospective study, 19 patients were subsequently ruled out from the statistic evaluation, i.e., Cox proportional-hazards regression analysis, of whom 10 belonging to the Abl group, six to the rate control and three to the rhythm control strategy group. The reasons for exclusion are represented as follows.
In the Abl group, for five patients the exclusion stemmed from the fact that the observation period had been too short (less than 6 months), whereas for other five patients it was caused by the fact that at least one severe comorbidity dominating the clinical scene was present (cancer in three cases, neurodegenerative disease in two cases).
In the rate control strategy group the reasons for the exclusion were the following: for four patients the presence of a brady-tachy syndrome which is known to contraindicate antiarrhythmic drugs, so that only anticoagulant therapy was practiced in these patients awaiting the implantation of a pace-maker. Furthermore, two patients had undergone atrioventricular node ablation with subsequent implantation of a device for cardiac resynchronization therapy.
In the rhythm control strategy group, three patients were excluded, due to intolerance to the pharmacological side effects.
In reality, this resizing of the database was carried out to make the three groups as homogeneous as possible, by excluding patients involved by clinical conditions or therapies unevenly distributed across the three groups, e.g., patients with sick sinus syndrome, all belonging to the rate control cohort, or patients with too short follow-up, all related to the Abl group. Thus, only 175 patients were included in the study. The follow-up mean duration in our retrospective study was 20 months (interquartile range = 14 - 24 months). Patients assigned to Abl were 74, of whom 21 females (Table 1 and Fig. 1). Besides, the group assigned to rate control strategy consisted of 60 patients, of whom 16 were female, whereas the group attributed to rhythm control strategy was composed of 41 patients, including 14 females (Table 1 and Fig. 1).
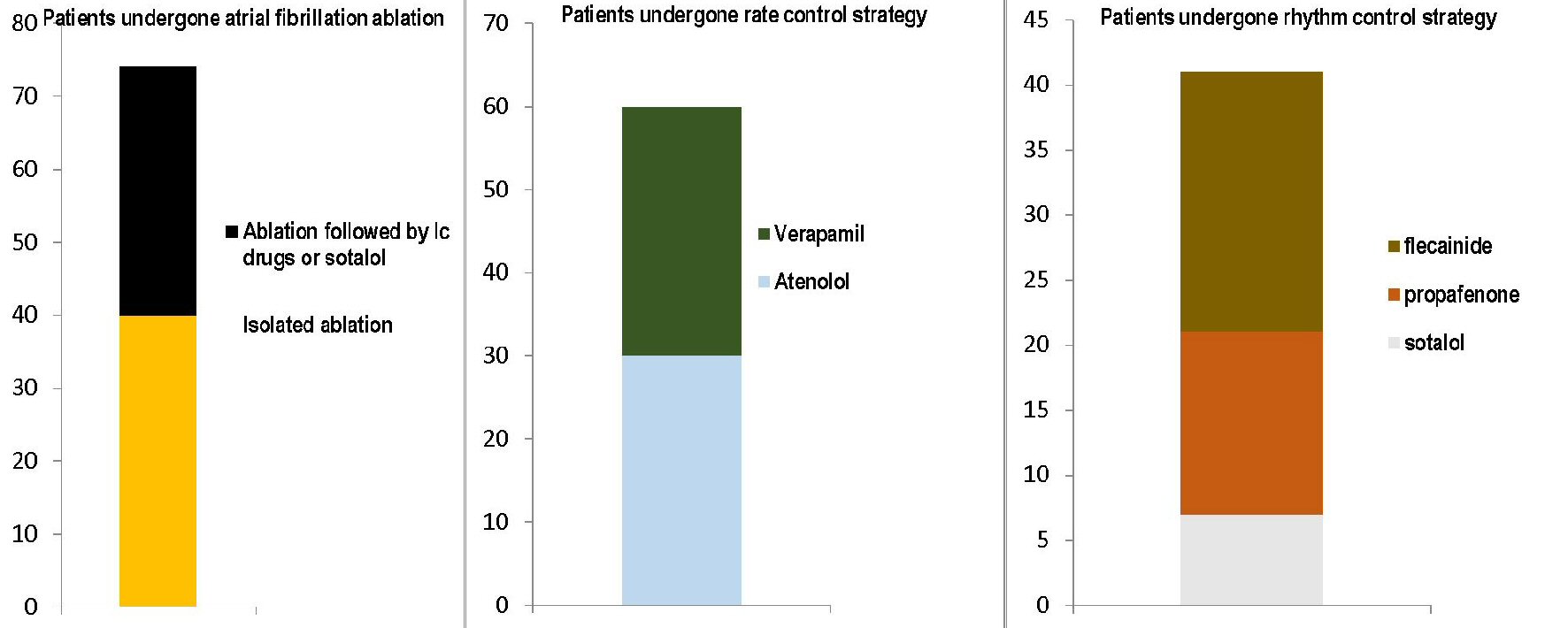 Click for large image | Figure 1. The figure shows the respective distributions of the three therapeutic approaches used in the series of 175 patients included in the present cohort retrospective study. The three graphs with stacked layers refer each to one of the three therapeutic modalities adopted: on the left, the transcatheter ablation, involving 74 patients, of whom 40 subjected to simple ablation and 34 with ablation followed by chronic administration of antiarrhythmics, namely IC drugs or sotalol; at the center, the rate control strategy with the use of atenolol (30 cases) or verapamil (30 cases); on the right the rhythm control strategy, with the bar consisting of three layers representing the use of sotalol (seven cases), propafenone (14 cases) and flecainide (20 cases), respectively. |
In accordance with this need to assure homogeneity between the groups, an attempt at selecting exclusively “standardized” therapies was made. In this regard, the patients were grouped in the three retrospective arms, by paying attention to gather only those for whom a well-defined drug dose was reported. Therefore, in the Abl group, constituted by 74 patients, of whom 40 subjected to simple Abl and 34 with Abl followed by chronic administration of antiarrhythmics (IC drugs, based on Vaughan Williams classification), or sotalol, the doses were as follows: propafenone (17 patients) 150 mg three times a day, flecainide (16 patients) 100 mg twice daily, sotalol (one patient) 80 mg twice daily. Moreover, patients belonging to rate control strategy group (n = 60) were treated as follows: 30 patients received atenolol (25 mg twice daily), while the remaining 30 were given verapamil (80 mg twice daily).
Conversely, patients who had been retrospectively enrolled in the rhythm control strategy group (n = 41), were given flecainide 100 mg twice daily (20 cases), propafenone 150 mg three times a day (14 cases) and sotalol 80 mg twice daily (7 cases) (Fig. 1).
During the abovementioned median follow-up, 25 patients experienced the primary composite endpoint (death, disabling stroke, severe bleeding and cardiac arrest).
The distribution of the primary endpoint in the three groups (Table 2) was as follows: 10 patients experienced the primary endpoint in the Abl group (n = 74), four patients fell under the primary endpoint in the rate control strategy group (n = 60), while 11 experienced this same outcome in the rhythm control strategy group (n = 41). The deaths were 13:7 due to irreversible progression of chronic heart failure (all belonging to Abl group), three due to myocardial infarction (one patient belonging to rate control and two to rhythm control strategy group), one due to pulmonary embolism consequent to deep vein thrombosis of the lower limbs (Abl group), one due to subarachnoid hemorrhage (rate control strategy group), and one caused by sepsis (rhythm control strategy group).
A Kaplan-Meier curve was built (Fig. 2) in order to graphically represent the different effect on the primary composite outcome exerted by each therapeutic regimen. A P-value of < 0.0001 (log-rank test) was found (Fig. 2), indicating a significantly decreased risk of the primary composite endpoint for patients assigned to the rate control strategy. In addition, a multivariate Cox proportional- hazards regression model was built (Table 3) by assuming as explanatory variables all those that, at the univariate Cox regression analysis preliminarily performed , had presented a sufficient predictive value, conventionally indicated by a P-value of ≤ 0.05. According to this criterion, the variables included in the model were: AF recurrences during follow-up, Abl (0 = isolated Abl; 1 = Abl followed by chronical administration of IC drugs or sotalol), rate control strategy (2 = atenolol; 3 = verapamil) , rhythm control strategy (4 = sotalol; 5 = propafenone; 6 = flecainide), time to first AF recurrence (days), anteroposterior diameter of left atrium (continuous variable), left ventricular ejection fraction (continuous variable), hypertension.
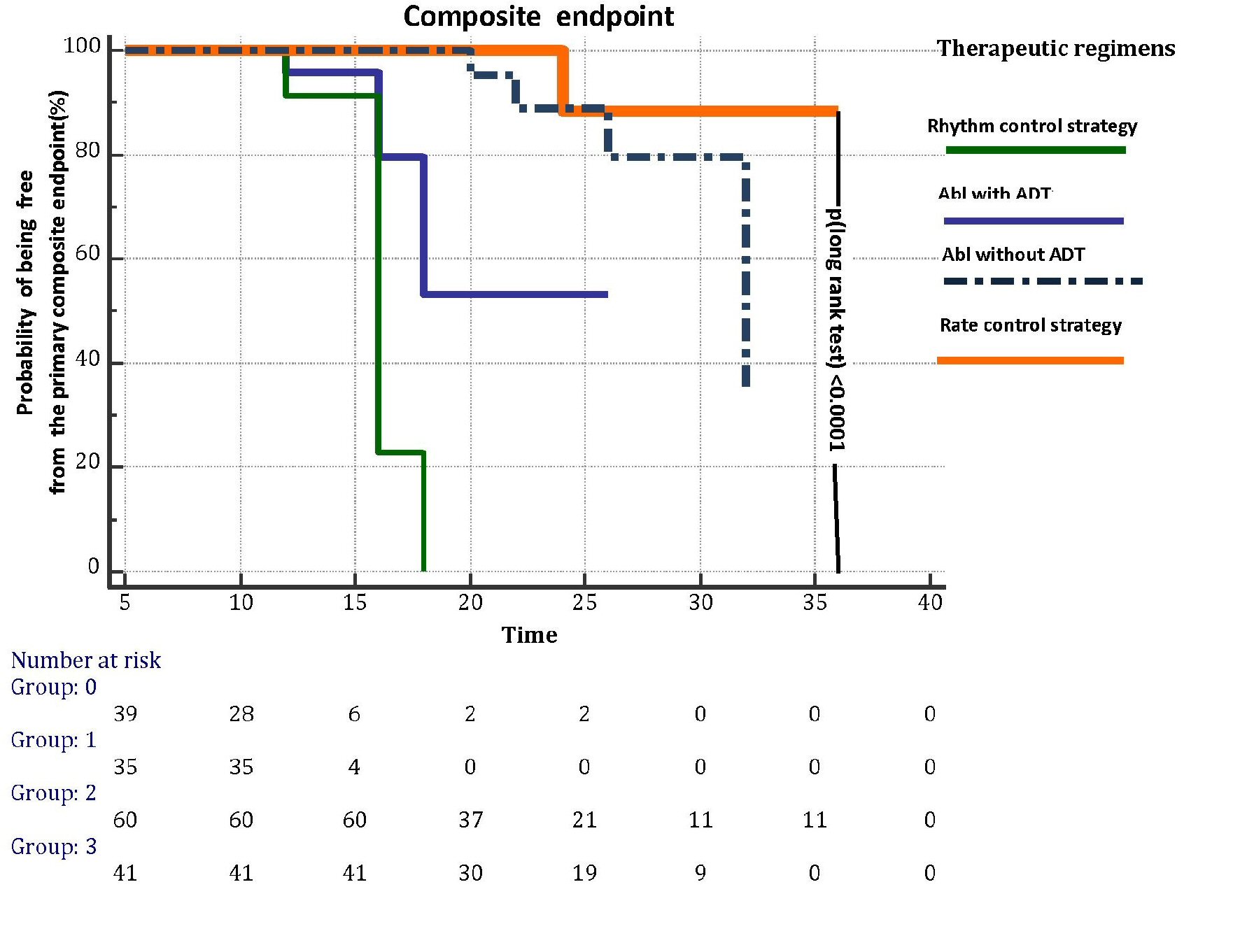 Click for large image | Figure 2. Kaplan-Meier curves illustrating the effects on primary composite endpoint, i.e., death, disabling stroke, major bleeding and cardiac arrest, exerted by each of the four therapeutic regimens for AF prevention (rhythm control strategy, abl with ADT, abl without ADT and rate control strategy) investigated in the retrospective study through a median follow-up of 20 months. Abl: transcatheter ablation; AF: atrial fibrillation; ADT: antiarrhythmic drug treatment. |
 Click to view | Table 3. Multivariable Cox Proportional-Hazards Regression Model |
An increased risk of being affected by the primary endpoint was associated with the rhythm control strategy (hazard ratio (HR): 3.3159; 95% CI: 1.5415 to 7.1329; P = 0.0023) and with AF recurrences during the follow-up (HR: 1.0448; 95% CI: 1.0020 to 1.0895; P = 0.0410). Even hypertension was associated with an increased risk (HR: 1.1040; 95% CI: 1.0112 to 1.9662; P = 0.0477). Conversely, rate control strategy was statistically significantly associated with a decreased risk of developing the primary endpoint (HR: 0.0711; 95% CI: 0.0135 to 0.3738; P = 0.0019). Abl was not a significant predictor of the primary endpoint. Likewise, the time (in days) to first AF recurrence did not predict the primary endpoint (Table 3).
Regarding the risk of AF recurrences in the comparison between the three antiarrhythmic therapeutic approaches (Abl, rate control strategy and rhythm control strategy), one-way ANOVA (Fig. 3) has highlighted important differences, i.e., a significantly lower frequency of AF recurrences has been found in the arm assigned to the rate control strategy. Importantly, any rise in mean ventricular response occurring at rest attaining 140 beats per minute and lasting no less than 30 min was arbitrarily equated to an AF relapse.
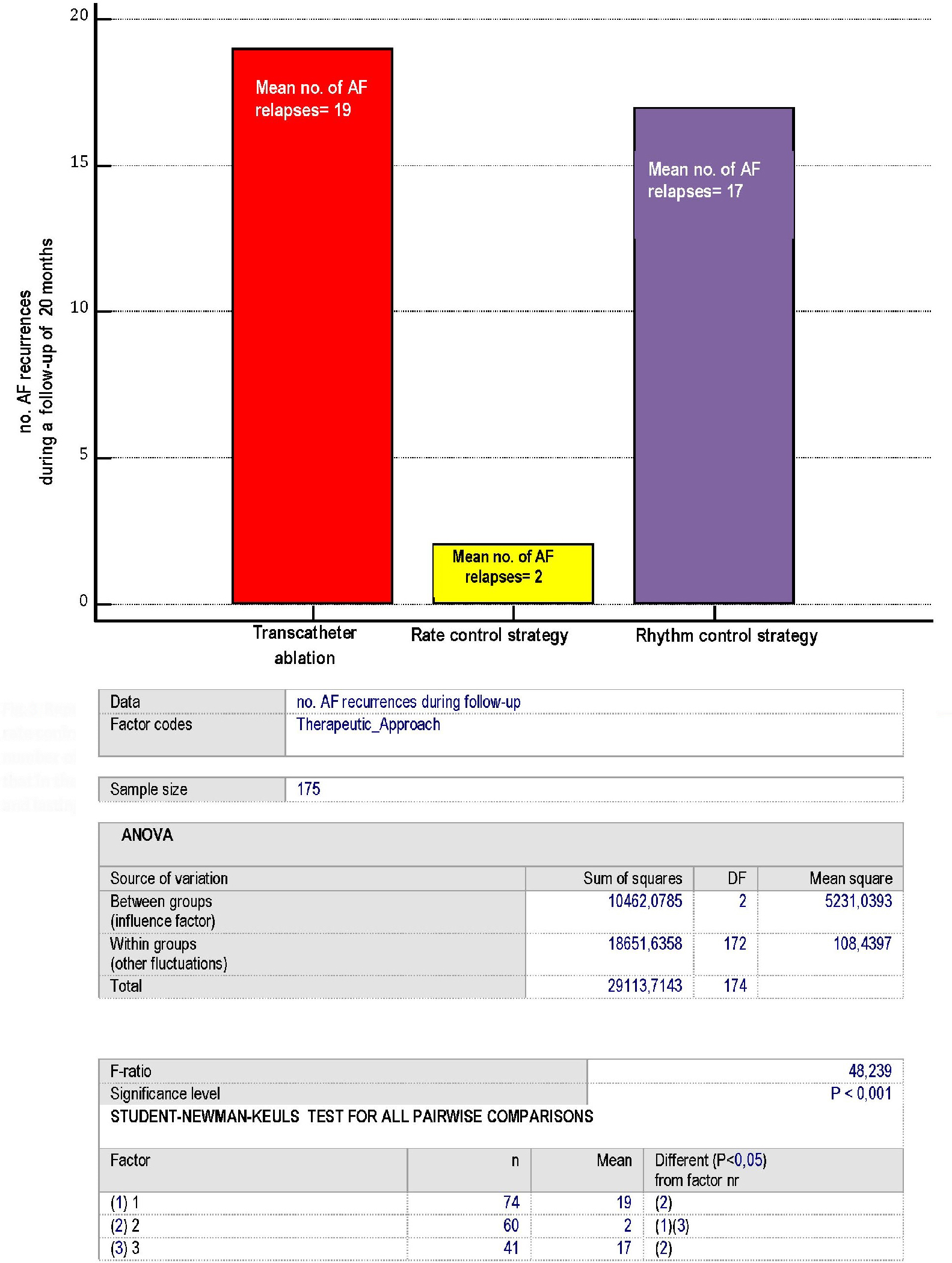 Click for large image | Figure 3. Representation of the AF recurrences, according to the type of therapeutic approach: whether consisting of ablation, rate control or rhythm control strategy. Please note that each of the numbers reported within the bars coincides with the mean number of AF recurrences identified for every patient of the three arms over a 20- month median follow-up. Moreover, note that in the rate control strategy group any rise in mean ventricular response occurring at rest attaining 140 beats per minute and lasting no less than 30 min was arbitrarily equated to an AF relapse. AF: atrial fibrillation. |
| Discussion | ▴Top |
Our results provide various ideas for reflection. The first aspect to consider is the fact that in our study a combined endpoint was chosen rather than the usual one of mortality. The choice of this composite was caused to a considerable extent by the relatively low number of cases of death in the recruited population. In other words, in order not to expand the time of the retrospective research, we have resorted to a combined endpoint. Furthermore, the variables “disabling stroke” and “severe bleeding” have been included in the composite due to the fact that they are the epiphenomena of a poorly managed anticoagulant therapy, a condition to which patients with pharmacologic AF therapy might be particularly exposed, especially when multiple challenging comorbidities are simultaneously present on the clinical scenario. Furthermore, the rationale to include the cardiac arrest in the composite coincides with the need to underline the risk, related to rhythm control strategy, of the occurrence of long QT and torsade de pointes, which are connected to increased risk of fatal or nonfatal cardiac arrest, as seen in patients undergone prolonged antiarrhythmic drug therapy. Because of these known drawbacks of the antiarrhythmic prophylaxis of AF relapses, Abl could represent a preferable alternative, and in fact a more favorable risk/benefit ratio has been recognized to Abl compared to pharmacological rhythm control strategies [7].
Recently, however, several randomized studies [8-10] have outlined that in some patients the remission of AF episodes is actually fleeting (even just a few weeks or a few months after the blanking period lasting 1 to 3 months), unless the operational protocol of abl does not provide for the chronic systematic administration of class IC or III drugs of the Vaughan Williams classification in the medium and long term. In reality, in ablated patients the current usual administration of class IC or class III drugs, to avoid possible AF recurrences, as well as the customary chronic anticoagulation, mostly warfarin, to prevent the cardio-embolic risk, has emerged as a resounding denial of the original axiom according to which abl would yield the advantage of exempting the patients from pharmacological therapies known for their pro-arrhythmic or pro-hemorrhagic potential. In truth, today, the model implemented in the majority of dedicated centers consists of abl followed by the administration of antiarrhythmics and warfarin not only in the peri- and post-procedural stages (blanking period), but indefinitely over time [11].
Another aspect to be emphasized, already mentioned in the Methods, is that the retrospective nature of the research might have generated biases. For example, we cannot rule out that patients who underwent abl were those with the most severe clinical picture. In reality, the recent societal guidelines provide that abl should be prescribed to patients severely disturbed by AF-related symptoms after at least one drug to treat AF has been tested [7]. It is therefore possible that the group of 74 ablated patients constituted a subset of patients with pharmacoresistant AF and/or with a more severe clinical picture. Thus, after a median follow-up of 20 months, the lack of a statistically significant improvement of the primary end-point in the ablated group compared to the rate control group might simply be the epiphenomenon of a more severe basal clinical profile, rather than being ascribed to a supposed inefficiency, ineffectiveness of Abl compared to rate control approach.
In the present retrospective study, while no statistically significant advantage regarding the primary endpoint has been identified for abl, the rate control strategy has been shown to have a protective value, in contrast to the rhythm control strategy which has exhibited a role of significant risk factor (Table 2). In our statistical model, namely multivariate Cox proportional-hazards regression analysis, AF recurrences have been a predictor of the primary composite endpoint of death, disabling stroke, major bleeding and cardiac arrest. Thus, it is plausible to infer the existence of an associative link between recurrent episodes of AF, and increased risk of exitus or serious adverse events (stroke, severe hemorrhage, cardiac arrest) in the mid-term (20 months of follow-up according to our experience).
 Click to view | Table 2. Distribution of the Four Components of the Primary Composite Endpoint Among the Four Therapeutic Regimens for Prevention of AF Recurrences |
Study limitations
The retrospective collection of a data set constitutes the most frequently used method for gathering medical-health information, but at the same time it implies an important limitation because it entails relatively frequent biases. This also applies to our study and should advise caution in the interpretation of the results. Further limitations of our study are the rather exiguous sample size and the relatively short duration of the follow-up. We hope that the conclusions reached can be confirmed by future prospective randomized studies with greater sample size.
Conclusions
In our retrospective cohort study, that included patients with paroxysmal, persistent or long-lasting persistent fibrillation, after a median follow-up of 20 months an increased risk of being affected by the primary endpoint (death, disabling stroke, major bleeding and cardiac arrest) was associated with the rhythm control strategy (P = 0.0023), as well as with AF recurrences during the follow-up (P = 0.0410), and history of hypertension (P = 0.0477). Conversely, rate control strategy was associated with a decreased risk of the primary endpoint (P = 0.0019).
Acknowledgments
None.
Conflict of Interest
The authors state that they do not have any conflicts of interest to declare.
Funding Disclosure
The authors state that the present manuscript has not benefitted from any source of funding.
| References | ▴Top |
- Jais P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, Hocini M, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118(24):2498-2505.
doi pubmed - Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659-666.
doi pubmed - Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, et al. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. 2000;102(21):2619-2628.
doi pubmed - Kottkamp H, Hindricks G, Borggrefe M, Breithardt G. Radiofrequency catheter ablation of the anterosuperior and posteroinferior atrial approaches to the AV node for treatment of AV nodal reentrant tachycardia: techniques for selective ablation of "fast" and "slow" AV node pathways. J Cardiovasc Electrophysiol. 1997;8(4):451-468.
doi pubmed - Wellens HJ. Should catheter ablation be performed in asymptomatic patients with Wolff-Parkinson-White syndrome? When to perform catheter ablation in asymptomatic patients with a Wolff-Parkinson-White electrocardiogram. Circulation. 2005;112(14):2201-2207; discussion 2216.
doi pubmed - Liu J, Sylwestrzak G, Barron J, Rosenberg A, White J, Whitney J, Redberg R, et al. Evaluation of practice patterns in the treatment of atrial fibrillation among the commercially insured. Curr Med Res Opin. 2014;30(9):1707-1713.
doi pubmed - Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962.
doi pubmed - Roux JF, Zado E, Callans DJ, Garcia F, Lin D, Marchlinski FE, Bala R, et al. Antiarrhythmics after ablation of Atrial Fibrillation (5A Study). Circulation. 2009;120(12):1036-1040.
doi pubmed - Darkner S, Chen X, Hansen J, Pehrson S, Johannessen A, Nielsen JB, Svendsen JH. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. 2014;35(47):3356-3364.
doi pubmed - Kaitani K, Inoue K, Kobori A, Nakazawa Y, Ozawa T, Kurotobi T, Morishima I, et al. Efficacy of antiarrhythmic drugs short-term use after catheter ablation for Atrial Fibrillation (EAST-AF) trial. Eur Heart J. 2016;37(7):610-618.
doi pubmed - Arbelo E, Brugada J, Hindricks G, Maggioni AP, Tavazzi L, Vardas P, Laroche C, et al. The atrial fibrillation ablation pilot study: a European Survey on Methodology and results of catheter ablation for atrial fibrillation conducted by the European Heart Rhythm Association. Eur Heart J. 2014;35(22):1466-1478.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


