| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 13, Number 2, April 2022, pages 104-109
Procedural Safety and Long-Term Clinical Outcomes in Patients Receiving Ultra-Long Everolimus-Eluting Stent: A Single-Center Real-World Experience
Nagendra Boopathy Senguttuvana, b, e, f, Rahul Kongaraa, e, Shanmugasundram Sadhanandhama, Nishok Victory Srinivasand, Santhosh Kumar Periyasamya, Balakrishnan Vinod Kumara, Ravi Shankar Pc, Meena Iyerd, Mahalakshmi Ramadossd, Vinodhini Subramaniana, Jayanthy Venkata Balasubramaniyana, Preetam Krishnamurthya, Sankaran Ramesha, Panchanatham Manokara, Thoddi Ramamurthy Muralidharana, Jayanthi Sathyanarayana Murthya, Sadagopan Thanikachalama
aDepartment of Cardiology, Sri Ramachandra Institute of Higher Education and Research (SRIHER), Porur, Chennai, Tamil Nadu 600116, India
bAdjunct Faculty, Department of Engineering and Design, Indian Institute of Technology-Madras, Chennai, India
cDepartment of Statistics, Sri Ramachandra Institute of Higher Education and Research (SRIHER), Porur, Chennai, Tamil Nadu 600116, India
dFaculty of Clinical Research, Sri Ramachandra Institute of Higher Education and Research (SRIHER), Porur, Chennai, Tamil Nadu 600116, India
eThese authors contributed equally to this article.
fCorresponding Author: Nagendra Boopathy Senguttuvan, Department of Cardiology, Sri Ramachandra Institute of Higher Education and Research (SRIHER), Chennai, India
Manuscript submitted January 19, 2022, accepted February 28, 2022, published online April 5, 2022
Short title: Clinical Outcomes in Patients Receiving ULEES
doi: https://doi.org/10.14740/cr1357
| Abstract | ▴Top |
Background: Diffuse long coronary lesions are difficult to treat percutaneously. The aim of the present study was to assess the procedural safety and long-term efficacy of the ultra-long (48-mm) drug-eluting stent Xience Xpedition.
Methods: This was an investigator-initiated, observational, all-comers study. A total of 92 patients with 93 lesions were enrolled in the study from October 2016 to October 2020. The primary outcome of the study was major adverse cardiac events (MACEs). Secondary outcomes were individual components of the primary outcome and procedural success.
Results: The mean (standard deviation (SD)) age of the participants was 58.8 (10.8) years. More than half of the patients had ST-segment elevation myocardial infarction (STEMI) at presentation (55.4%). Ten patients were in cardiogenic shock (CGS; 10.8%). Most of the lesions were located in the left anterior descending artery (48.3%). American College of Cardiology/American Heart Association (ACC/AHA) type C was the most common lesion type amongst the intervened vessels (46.74%), with a mean syntax score (SD) of 16.99 (8.89). The mean stent diameter used was 2.77 mm (0.25). MACE was observed in 7.6% of patients studied at a median follow-up of 24 months. MACE was significantly lower in the population without CGS, occurring in only 2.4% of the patients; a significant difference in MACE was observed in patients with and without CGS (P < 0.001). Procedural success was obtained in 89.2% of total population; however, 96.3% of patients without CGS had procedural success.
Conclusions: The deployment of the ultra-long 48-mm Xience Xpedition stent is feasible, safe, and effective; and it was associated with a good intermediate-term clinical outcome.
Keywords: 48-mm stent; Xience; Percutaneous coronary intervention; Coronary artery disease
| Introduction | ▴Top |
Diffuse coronary artery lesions account for 20% of all percutaneous coronary intervention (PCI) [1]. Many such complex lesions are addressed percutaneously, leading to an increasing need for techniques to handle them. For example, the use of longer balloons followed by deployment of longer stents or multiple stents with overlapping segments. However, stent overlap results in increased neointimal proliferation, lumen loss due to delayed healing, disparity in drug deposition, and increased inflammation. Furthermore, the overlapping portions make the vessel rigid due to excess metal, which may lead to stent fracture and cause further vascular injury, leading to a higher incidence of restenosis [2]. The length of the stents (drug-eluting stents (DES)) is directly proportionally to the incidence of in-stent restenosis [3]; a study done by Cassese et al showed that lesion length was independently associated with restenosis (odds ratio (OR): 1.27; 95% confidence interval: 1.21 - 1.33, for every 10 mm increase in the length of the stent) [4].
Everolimus-eluting stents (EESs) are more effective than old-generation DES when used in long lesions [5]. Until recently, the longest stent approved by the US Food and Drug Administration (FDA) was the 38-mm DES (stents greater than or equal to 38 mm are considered ultra-long stents). However, a 48-mm EES with platinum-chromium platform with an absorbable polymer has now been approved. The Drugs Controller General of India (DCGI) has approved the 48-mm Xience Xpedition stent (Abbott Vascular, Santa Clara, California). However, there is no long-term data available regarding the clinical outcomes of the ultra-long 48-mm Xience Xpedition stent. In this study, we analyzed the intermediate-term clinical outcomes and immediate procedural success of this device.
| Materials and Methods | ▴Top |
Study design
This was an investigator-initiated, observational, all-comers study. The present study included all consecutive patients who had undergone PCI using the 48-mm Xience Xpedition stent from October 2016 to October 2020 in the Department of Cardiology, Sri Ramchandra Institute of Higher Education and Research (SRIHER). Xience Xpedition (48 mm) is an L-605 cobalt-chromium alloy stent pre-mounted on a rapid exchange delivery system. It is available in different sizes (2.5 mm, 2.75 mm, 3.00 mm, and 3.5 mm). The abluminal surface of the device is coated with a thin layer of durable fluoropolymer, which is used as the carrier for everolimus, at a concentration of 100 µg/mm2 [6].
The details of de-identified patients were collected from the Cardiac Catheterization Registry and patient database software (Health Management Information System - HMIS, SRIHER). Baseline characteristics of the patients, as well as angiographical and interventional details, were recorded. The recruited patients were contacted during the follow-up by the respective physicians or by the second author. There was no interference in the routine care of the included patients.
Informed consent was obtained from all participants, and the patients who did not give consent were excluded from the study. The study was approved by the institute’s ethics committee. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Outcomes
The primary outcome of the study was major adverse cardiac events (MACEs) which consisted of cardiovascular death, nonfatal myocardial infarction (MI), and repeat intervention in the target vessel.
Cardiovascular death was defined according to the definitions developed by the American College of Cardiology/American Heart Association (ACC/AHA) task force for cardiovascular endpoints in clinical trials [7].
Nonfatal MI was defined by the elevation of cardiac troponin (cTn) values five times the 99th percentile upper reference limit (URL) in patients with normal baseline values as per the fourth universal definition of MI [8]. Similarly, reintervention included target vessel revascularization.
Secondary outcomes included individual components of the composite primary outcome and procedural success, which was defined as the successful deployment of the stent without any procedural complications such as cardiac death, dissection, and stent thrombosis. In addition, we also assessed admission for heart failure, stroke, cardiovascular procedures, and other cardiovascular causes.
Statistical analysis
Descriptive data were expressed in terms of ratio, proportion, or percentage. Mean and median values (interquartile range) were used for discrete quantitative data. Continuous variables were analyzed by the t-test. Categorical variables were analyzed by the Chi-squared test. A Kaplan-Meier curve survival analysis was performed. A P value < 0.05 was considered significant. SPSS Version 20 (IBM) was used for statistical analysis.
| Results | ▴Top |
A total of 92 patients with 93 lesions were enrolled in the present study, with a median follow-up of 24 months. Baseline characteristics of the patients are described in Table 1. The mean (standard deviation (SD)) age of the participants was 58.8 (10.8) years; 26.1% of the participants were female. More than half of the patients had ST-segment elevation myocardial infarction (STEMI) at presentation (55.4%). The mean ejection fraction (SD) was 51% (0.1%). Ten patients in the study population were in cardiogenic shock (CGS; 10.87%).
 Click to view | Table 1. Baseline Characteristics and Angiographic and Interventional Details of the Patient Population |
The left anterior descending (LAD) artery was the most intervened vessel (48.38%), followed by the right coronary artery (RCA) (45.18%), left circumflex (LCX) (3.22%), and left main (LM) (3.22%). Lesions were classified based on the ACC/AHA guidelines; all lesions were ACC/AHA type C lesions. Approximately 11% (n = 10) of patients had severely calcified lesions, and 20.7% (n = 19) had severe tortuosity in the vessels. Two-thirds of the patients (66.30%) had multivessel involvement. The mean syntax score (SD) of the patients was 16.99 (8.89). The Xience Xpedition 48-mm stent was used for primary PCI in 25% (n = 23) of patients. The mean diameter (SD) of the stent used was 2.77 (0.25) mm. Most of the lesions were predilated (98.91%) with semicompliant or noncompliant balloons. The ultra-long stents were post-dilated in 88.04% of patients with noncompliant balloons. Rotational atherectomy and image guidance using intravascular ultrasonogram were employed in 2.17% of patients. All the patients received dual antiplatelet therapy (DAPT) for a minimum duration of 12 months with all patients receiving aspirin 75 mg and the majority receiving ticagrelor (82.61%, n = 76).
Outcomes
The primary outcome of the study, MACE, was observed in 7.61% (n = 7) of patients at a median follow-up of 24 months. MACE occurred in five patients who had CGS (50%) as compared with those without CGS (2.4%, n = 2, P ≤ 0.001) The significant difference regarding the occurrence of MACE between the CGS and non-CGS groups was influenced by death at admission.
A total of eight deaths were reported in the present study population (8.7%), of which five deaths occurred in the CGS group. Out of the five patients who died with CGS, four patients (three of which had multivessel disease and one which had single-vessel disease) presented with acute MI and severe left ventricular (LV) dysfunction (ejection fraction less than 30%), and were taken up for primary PCI. LAD was the culprit lesion in three patients, and RCA was the culprit lesion in one patient. These patients had refractory CGS with multiple organ dysfunction syndrome and succumbed to their illness within 24 h of the procedure. The fifth patient had undergone a procedure with the Xience Xpedition 48-mm stent in 2016 and had been doing relatively well till 2018 when he developed severe LV dysfunction CGS and succumbed to his illness. Three patients who did not have CGS died during the follow-up. Two of those three patients were doing well for 1 year after stent implantation but had a possible out-of-hospital cardiac death, and one patient had a noncardiac cause of death after colon surgery for carcinoma of the colon. Nonfatal MI of the target vessel and target vessel revascularization were not seen in any of the patients. Non-target vessel revascularization was seen in 4.35% (n = 4) of patients.
Procedural success was seen in 89.2% of the overall population and in 96.3% of patients without CGS. As mentioned in Table 2, a total of nine patients developed complications such as dissection (n = 4), intraprocedural stent thrombosis (definitive stent thrombosis; n = 1), and immediate cardiac death (n = 4). All the patients who died had CGS. Also, the patient who had intraprocedural stent thrombosis was also in CGS. He presented with STEMI with CGS; he had definitive evidence of stent thrombosis (1.08%) as he developed chest pain after the procedure. An immediate angiogram revealed stent thrombosis due to edge dissection. This was addressed by placing an additional stent. A total of four patients had dissections (4.34%) within 3 mm of the stented segment in the LAD. All of them were treated with an additional stent. The procedural success is significantly higher in patients without CGS.
 Click to view | Table 2. Outcomes of Patients Who Received the Ultra-Long 48-mm Xience Xpedition Stent |
Kaplan-Meier survival curve analysis
We performed a Kaplan-Meier curve analysis to analyze survival in the patient groups. As expected, the survival was lower in patients with CGS compared to those without CGS (Fig. 1). We observed a step-down in the survival in the non-CGS group between 18 and 20 months; however, we did not find any further events till the end of 60 months.
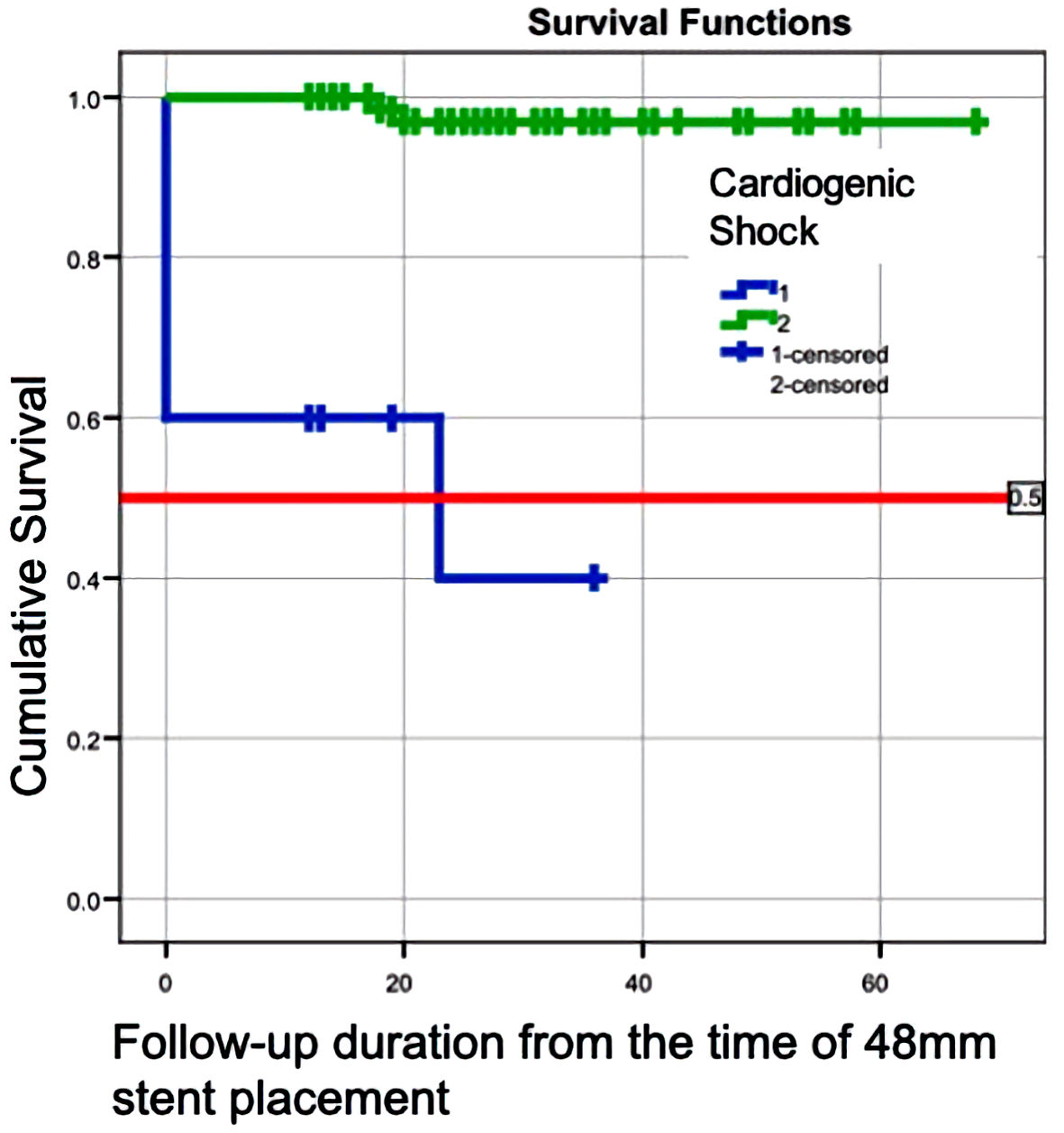 Click for large image | Figure 1. Kaplan-Mayer curve showing the survival in patients with cardiogenic shock and without cardiogenic shock. |
| Discussion | ▴Top |
In our all-comers study, we found that the intermediate-term clinical outcome of Xience Xpedition 48-mm ultra-long stent was very good. The ultra-long 48-mm Xience Xpedition stent was effective even when used during acute coronary events. The strengths of our study are its prospective design, which included all-comers with variable clinical presentation, and the at least intermediate-term follow-up period.
The incidence of long coronary lesions has increased with the increased prevalence of comorbid conditions [9]. Previously, patients with such lesions were excluded from clinical studies. Finn et al performed animal studies to identify the arterial reaction to first-generation DES vs. bare-metal stents (BMS) [10]. First-generation DES demonstrated delayed healing and promoted inflammation at the overlap sites when compared with the BMS. More luminal heterophils/eosinophils and fibrin deposition were observed with Taxus stents than Cypher stents [10]. Second-generation DES, such as Xience (Abbott Vascular, Santa Clara, California), Promus (Boston Scientific, MA, USA), and Endeavor Resolute (Medtronic, Minneapolis, USA), have thinner stent struts, and have more biocompatible polymers compared with first-generation DES. Such technological advancements have resulted in a reduction in vascular injury, inflammation and rapid endothelialization [11]. In the porcine studies done by Farooq et al, longer lesions intervened with overlapping everolimus stent platforms have shown to have good overlap endothelialization within 30 days [12].
A meta-analysis involving 13,266 patients undergoing PCI for very long lesions (> 35 mm) with overlapping stent treatment with the EES (XIENCE V) from six trials (Spirit II, III, IV, V, Spirit Small Vessel, and XIENCE V USA) demonstrated that there was no difference in outcomes such as target lesion failure (8.9% vs. 10%; P = 0.63) and MACE (9.2% vs. 10%; P = 0.74) compared to the control group comprising lesions > 24 to < 35 mm. The longest stent used in these studies was a 38-mm stent. They concluded that XIENCE V (EES) appeared safe and effective for PCI for long lesions [13]. All the above-described studies used an EES of size less than or equal to 38 mm, and longer lesions were addressed by an overlapping stenting technique.
The only trial available till date for the treatment of very long coronary lesions with a single EES (Xience Xpedition 48-mm stent) was performed by Tan et al [6]. A total of 123 patients were followed up for 12 months. The study included patients presenting with chronic coronary syndrome (CCS) (n = 51) and acute coronary syndrome (ACS) (n = 72). The included patients presented with a wide spectrum of coronary lesions, with the majority belonging to ACC/AHA type C with bifurcations (n = 71), heavy calcifications (n = 46), significant tortuosity (n = 15), and severe LV dysfunction (left ventricular ejection fraction (LVEF) < 30%, n = 37). The procedural success rate was 99%, the 30-day MACE rate was 0.8% and the 12-month MACE was 3.3%.
Compared with their studies, we found that procedural success was seen in 89.2% of the overall population and in 96.3% of patients without CGS. This is likely due to the inclusion of sicker patients with CGS in the present study. In the study by Tan et al [6], 38.9% of patients had STEMI, whereas 55.4% had STEMI in our study. Additionally, more than three-fourths of the patients in our study had ACS. These differences in findings may also be attributed to ethnic differences. Most importantly, the definition of procedural success was different in our study compared with the aforementioned study, which defined procedural success as “delivery of the stent with the attainment of < 30% stenosis without in-hospital MACE”. On the other hand, we defined procedural success as “successful deployment of the stent without any procedural complications, including cardiac death, dissection, and stent thrombosis”. Four of our patients had dissections that could have affected our results. These differences might have affected the procedural outcomes in our study.
Longer follow-up is an added strength of our study. However, we observed lower clinical events in our study after a month of the procedure, compared with the above study. The potential reasons could be better risk factor control, a younger subset of patients, performance of aggressive post-dilatation in nearly all patients (88.04%), or confining ourselves to identify clinical restenosis without looking for angiographic binary stenosis.
Like the published literature on MI and CGS, where the in-hospital mortality varies between 40% and 60%, we have noticed higher MACE and lower procedural success in patients with CGS, with 50% in-hospital mortality in our study [14].
Limitations
The sample size of the study was small. However, we have data regarding extended follow-up of the patients, and have included patients with a wide variety of coronary anatomies and clinical presentations. Also, it is a single-center study; whether the results are applicable to other centers is questionable.
Conclusions
On the basis of our all-comers study, which included a small sample size, we conclude that the deployment of the ultra-long Xience Xpedition 48-mm stent is feasible, safe, and effective with a good intermediate-term clinical outcome.
Acknowledgments
We thank all the patients and staff members of the Cardiac Care Center, SRIHER, Chennai.
Financial Disclosure
This study did not receive any funding in any form.
Conflict of Interest
The authors declare that there is no conflict of interest.
Informed Consent
Informed consent was obtained from all participants
Author Contributions
NBS: conceptualization, designing, interpretation supervision, writing of original draft, review and editing. RK: execution, interpretation, review and editing. Ravi Shankar P: statistics. SS, NVS, SKP, BVK, MI, MR, VS, JVB, PK, SR, PM, TRM and JSM: reviewing and editing. ST: guarantor, reviewing and editing.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Kobayashi Y, De Gregorio J, Kobayashi N, Akiyama T, Reimers B, Finci L, Di Mario C, et al. Stented segment length as an independent predictor of restenosis. J Am Coll Cardiol. 1999;34(3):651-659.
doi - Jurado-Roman A, Abellan-Huerta J, Requena JA, Sanchez-Perez I, Lopez-Lluva MT, Maseda-Uriza R, Piqueras-Flores J, et al. Comparison of clinical outcomes between very long stents and overlapping stents for the treatment of diffuse coronary disease in real clinical practice. Cardiovasc Revasc Med. 2019;20(8):681-686.
doi pubmed - Mauri L, O'Malley AJ, Popma JJ, Moses JW, Leon MB, Holmes DR, Jr., Teirstein PS, et al. Comparison of thrombosis and restenosis risk from stent length of sirolimus-eluting stents versus bare metal stents. Am J Cardiol. 2005;95(10):1140-1145.
doi pubmed - Cassese S, Byrne RA, Tada T, Pinieck S, Joner M, Ibrahim T, King LA, et al. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart. 2014;100(2):153-159.
doi pubmed - Claessen BE, Smits PC, Kereiakes DJ, Parise H, Fahy M, Kedhi E, Serruys PW, et al. Impact of lesion length and vessel size on clinical outcomes after percutaneous coronary intervention with everolimus- versus paclitaxel-eluting stents pooled analysis from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) and COMPARE (Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice) Randomized Trials. JACC Cardiovasc Interv. 2011;4(11):1209-1215.
doi pubmed - Tan CK, Tin ZL, Arshad MKM, Loh JKK, Jafary FH, Ho HH, Ong PJL, et al. Treatment with 48-mm everolimus-eluting stents: procedural safety and 12-month patient outcome. Herz. 2019;44(5):419-424.
doi pubmed - Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on clinical data standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol. 2015;66(4):403-469.
doi pubmed - Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, et al. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138(20):e618-e651.
doi pubmed - Mehran R, Dangas G, Mintz GS, Lansky AJ, Pichard AD, Satler LF, Kent KM, et al. Atherosclerotic plaque burden and CK-MB enzyme elevation after coronary interventions : intravascular ultrasound study of 2256 patients. Circulation. 2000;101(6):604-610.
doi pubmed - Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, Tefera K, Skorija K, et al. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation. 2005;112(2):270-278.
doi pubmed - Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich KL, Giddings VL, Coleman L, et al. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123(13):1400-1409.
doi pubmed - Farooq V, Serruys PW, Heo JH, Gogas BD, Onuma Y, Perkins LE, Diletti R, et al. Intracoronary optical coherence tomography and histology of overlapping everolimus-eluting bioresorbable vascular scaffolds in a porcine coronary artery model: the potential implications for clinical practice. JACC Cardiovasc Interv. 2013;6(5):523-532.
doi pubmed - Bouras G, Jhamnani S, Ng VG, Haimi I, Mao V, Deible R, Cao S, et al. Clinical outcomes after PCI treatment of very long lesions with the XIENCE V everolimus eluting stent; Pooled analysis from the SPIRIT and XIENCE V USA prospective multicenter trials. Catheter Cardiovasc Interv. 2017;89(6):984-991.
doi pubmed - Elgendy IY, Van Spall HGC, Mamas MA. Cardiogenic shock in the setting of acute myocardial infarction: history repeating itself? Circ Cardiovasc Interv. 2020;13(3):e009034.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


