| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 14, Number 6, December 2023, pages 446-452
The Heart of Rett Syndrome: A Quantitative Analysis of Cardiac Repolarization
Michael P. Collinsa, b, Mark C. Johnsona, Robin C. Rytherc, Judith L. Weisenbergb, Peter T. Heydemannd, Colleen M. Buhrfiendd, William A. Scotte, Dallas M.M. Armstronge, Haley M. Kerne, Hoang H. Nguyend, e, f
aDepartment of Pediatrics, Washington University in St. Louis School of Medicine, St. Louis, MO, USA
bDepartment of Pediatrics, University of Rochester School of Medicine and Dentistry, Rochester NY, USA
cDepartment of Neurology, Washington University in St. Louis School of Medicine, St. Louis, MO, USA
dDepartment of Pediatrics, Rush University Medical College, Chicago, IL, USA
eDepartment of Pediatrics, The University of Texas Southwestern Medical Center, Dallas, TX, USA
fCorresponding Author: Hoang H. Nguyen, Department of Pediatrics, The University of Texas Southwestern Medical Center, Dallas, TX 75390, USA
Manuscript submitted September 22, 2023, accepted October 20, 2023, published online November 3, 2023
Short title: Cardiac Repolarization Abnormalities in RTT
doi: https://doi.org/10.14740/cr1580
| Abstract | ▴Top |
Background: Rett syndrome (RTT) is a developmental encephalopathy disorder that is associated with a high incidence of sudden death presumably from cardiorespiratory etiologies. Electrocardiogram (ECG) abnormalities, such as prolonged heart-rate corrected QT (QTc) interval, are markers of cardiac repolarization and are associated with potentially lethal ventricular arrhythmias. This study investigates the cardiac repolarization characteristics of RTT patients, including QTc and T-wave morphology characteristics.
Methods: A retrospective quantitative analysis on 110 RTT patients and 124 age and sex-matched healthy controls was conducted.
Results: RTT patients had longer QTc, more abnormal T-wave morphology, and greater heterogeneity of cardiac repolarization parameters compared to controls. Even RTT patients without prolonged QTc had more abnormal ECG and T-wave characteristics than controls. Among RTT patients, MECP2 patients had prolonged QTc compared to CDKL5 and FOXG1 patients. A subset of five RTT patients who died had normal QTc, but more abnormal T-wave morphology than the remaining RTT patients.
Conclusions: Cardiac repolarization abnormalities are present in RTT patients, even without long QTc. T-wave morphology is related to RTT genotype and may be predictive of mortality. These findings could be used to help the management and monitoring of RTT patients.
Keywords: Rett syndrome; Long QT; Abnormal T wave; T-wave analysis; Electrocardiogram; Cardiac repolarization; Ventricular repolarization
| Introduction | ▴Top |
Rett syndrome (RTT) is an X-linked developmental encephalopathy with a prevalence of about 1 in 10,000 females [1]. Both typical RTT and atypical RTT fall under the term RTT. Mutations of MECP2 are found in most patients. Atypical RTT encompasses monogenic disorders such as FOXG1 syndrome, CDKL5 deficiency disorder, MECP2 duplication syndrome, and MECP2-related severe neonatal encephalopathy as well as other developmental encephalopathies associated with pathogenic variants in GABBRG2, NTNG1, SMC1A, and MEF2C [2, 3].
RTT patients have an overall mortality rate of 1.2% per year, of which 20-26% are sudden and unexpected, and up to 35% are suspected to be cardiorespiratory arrests. The etiologies of cardiorespiratory arrest have yet to be fully elucidated. Leading potential causes include seizure (i.e., sudden unexpected death in epilepsy), autonomic dysfunction, or cardiac arrhythmias [4].
Multiple cardiac abnormalities have been associated with RTT including subclinical biventricular myocardial dysfunction, reduced heart rate (HR) variability, cardiac arrhythmias, and abnormal cardiac repolarization on electrocardiogram (ECG) (such as prolonged heartrate corrected QT (QTc) interval and nonspecific T-wave abnormalities) [5-11]. Since prolonged QTc and increased heterogeneity of cardiac repolarization are associated with an increased risk of life-threatening ventricular arrhythmias, they may be associated with sudden death in RTT [12, 13]. However, QTc measurements are variable in RTT while T-wave abnormalities have only been qualitatively described as nonspecific in one small case series of RTT [9, 14]. Automated identification of electrocardiographic features of abnormal cardiac repolarization may be important for risk stratification and surveillance of RTT patients. We sought to compare quantitative morphological features of the T wave including flatness, asymmetry, and notching between RTT patients and normal controls. We also investigated the relationship of these morphologic measures and QTc with genotype and mortality in RTT patients.
| Materials and Methods | ▴Top |
Patients
This study was approved by the Institutional Board Review at Washington University School of Medicine and Rush Medical College. Informed consent was waived by the Institutional Board Review at Washington University School of Medicine and Rush Medical College. All research was performed in accordance with the Declaration of Helsinki. Female RTT patients at St. Louis Children’s Hospital and Rush University Children’s Hospital, who had at least one ECG performed from January 2006 to January 2020 were included. Medical records were reviewed to obtain demographic, genotype, and mortality data. None of the patients received anti-arrhythmic medications. Details regarding all other medications at the time of ECG were not available from the chart review. The age and sex-matched controls were patients referred to the Cardiology Clinic at St. Louis Children’s Hospital for chest pain or syncope, who subsequently were found to have no cardiac disease.
ECG analysis
We retrospectively analyzed ECGs of clinical female RTT patients obtained from St. Louis Children’s Hospital and Rush University Children’s Hospital, which were collected between January 2006 and January 2020. Both institutions utilize MUSE Cardiology Information System (GE Healthcare, Waukesha, WI) to record and interpret ECGs. The QTGuard Plus Software (GE Healthcare, Milwaukee, WI) was used to analyze the digital ECG files, determine the QTc according to Bazett’s formula (QT divided by the square root of the previous RR interval, QT/√RR; ms), and generate morphology parameters related to flatness, asymmetry, and notching of the T wave as previously described [15]. A QTc ≥ 460 ms was considered prolonged. A median beat for each lead of the surface 12-lead ECG was formed. These median beats were then transformed to median beats on XYZ orthogonal leads. The principal component analysis (PCA) was then applied to the ST-T segment of the XYZ median beats forming orthogonal PCA leads describing the spatial direction of the ST-T loop. As a result, there are three PCA leads used for analysis. The T wave of the first PCA lead is a geometrical representation of the optimal projection of the heart dipole vector during the ST-T segment. This lead was used to derive the flatness, asymmetry, and notch score of the T wave for each ECG. The flatness score is based on the inverse kurtosis of the area unit of the T wave with a higher score corresponding to a flatter T wave. The asymmetry score is based on the difference in the slope and duration of the ascending and descending limbs of the T wave. The lower the asymmetry score, the more symmetric the T wave. The presence of notching is based on the radius of curvature of the T wave, where values > 0 correspond with the presence of notching. The morphology combination score (MCS) was calculated by summing the three morphology scores with the coefficient of each score determined by linear regression; flatness had the highest coefficient, and a higher score indicates more abnormal morphology (Fig. 1). The heterogeneity of repolarization was represented by the principal component analysis ratio 2 (PCA-2) defined as the ratio of the second PCA lead to the first PCA lead. A higher PCA-2 indicates increased cardiac repolarization heterogeneity. Other ECG parameters determined by QT Guard Plus Software include HR (beats per minute (bpm)), QT interval, heart-rate corrected QT interval using Bazett’s formula, time from the J point to the peak of the T wave (JTp, ms), and time from the peak of the T wave to the end of the T wave (TpTe, ms) (Fig. 1).
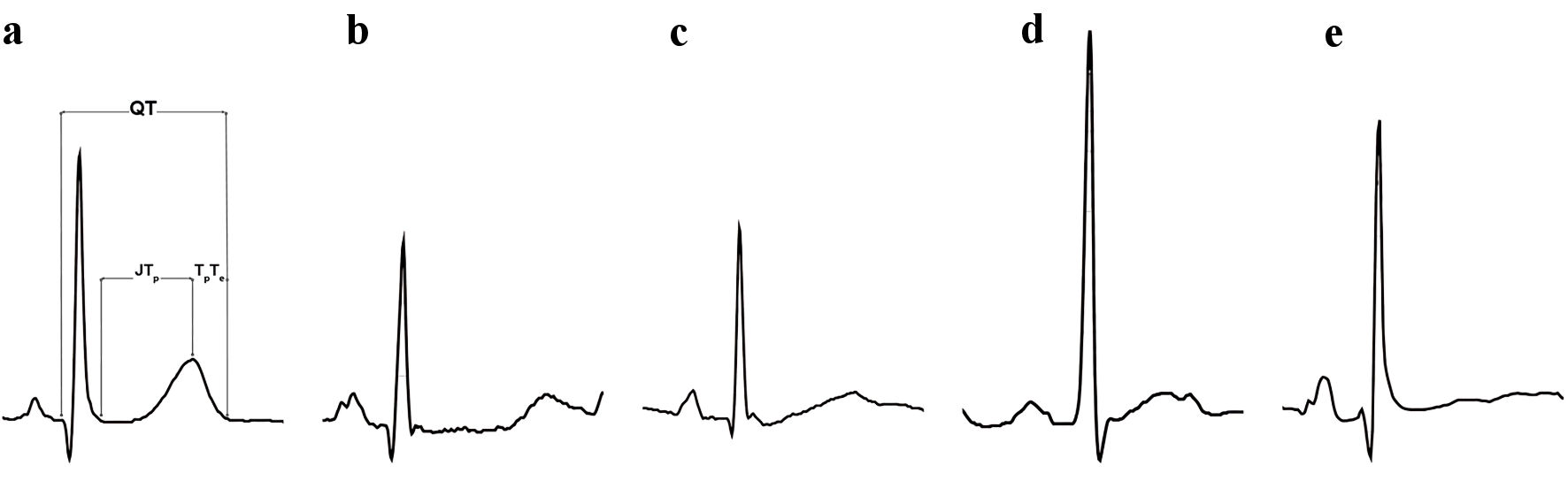 Click for large image | Figure 1. Depiction of the electrocardiographic measurements along with T-wave morphology characteristics seen in the study’s RTT patients. (a) Normal T wave. (b) Prolonged QT with prolonged JTp. (c) Asymmetric T wave. (d) Notched T wave. (e) Flat T wave. This ECG had the highest morphology combination score (MCS). ECG: electrocardiogram; RTT: Rett syndrome; JTp: time from the J point to the peak of the T wave. |
Statistical analysis
All values were reported as means and standard deviations (SDs). Continuous variables were compared using a Mann-Whitney U test for two group comparisons. A Chi-square test or Fisher’s exact test was used to compare proportions. ECG and T wave characteristics, due to repeat measurements per subject, were modeled using mixed random effect models with heterogeneous variance structure. Fixed effects comprised RTT status, genotype, mortality status, age, long QT status, and the interactions between RTT status and long QT status. Random effects included subjects. Degrees of freedom were adjusted with Kenward-Roger’s method. P values for post-hoc pair-wise comparisons were adjusted using Tukey’s method. Analyses were performed in SAS (Version 9.4; SAS Institute, Cary, NC).
| Results | ▴Top |
Baseline characteristics
A total of 110 unique female RTT patients with 577 ECGs were analyzed. The mean age of the RTT group was 9.3 (SD = 5.2) years. Seventy-eight patients (71%) had MECP2 mutations, eight patients (7%) had CDKL5 mutations, and three patients (3%) had FOXG1 mutations. The remaining 21 patients (19%) were diagnosed clinically; they either did not have genetic testing results available or they did not have known pathogenic RTT mutations. There were five deaths (5/110, 5%) in the RTT group. Ages at death ranged from 12 to 36 years. Two of the mortalities had MECP2 mutations, while the remaining three patients were classified as clinical RTT. One deceased patient had multiple ECGs with long QTc, but the cause of death was respiratory failure. The cause of death of the other four patients could not be determined. The control group comprised 124 subjects, who had 140 ECGs (Table 1).
 Click to view | Table 1. Baseline and Electrocardiographic Characteristics |
Electrocardiographic characteristics
All groups had normal QRS duration. RTT patients had a higher HR than controls. They also had longer QTc intervals compared to controls (445.6 (SD = 31.6) ms vs. 422.8 (SD = 19.3) ms; P < 0.001). The MECP2 group had the longest mean QTc (448.1 ms (SD = 32.8 ms)) (Table 1). Fifty-five (50%) RTT patients had a prolonged QTc on at least one ECG. Forty-five of these patients (82%) had MECP2 mutations. These 45 patients constituted 41% (45/11) of the RTT group. Thirty-eight patients (38/110, 35%) had two or more ECGs with long QTc. Figure 2 details the percentage of RTT patients with repeated prolonged QTc on ECG. Among the common MECP2 mutations (R106W, R133c, R168X, R255X, R270X, R294XX, R306C, R306H, T158M), there was no difference in the proportion of patients with repeated prolonged QTc on ECG (P = 0.37) (Table 1).
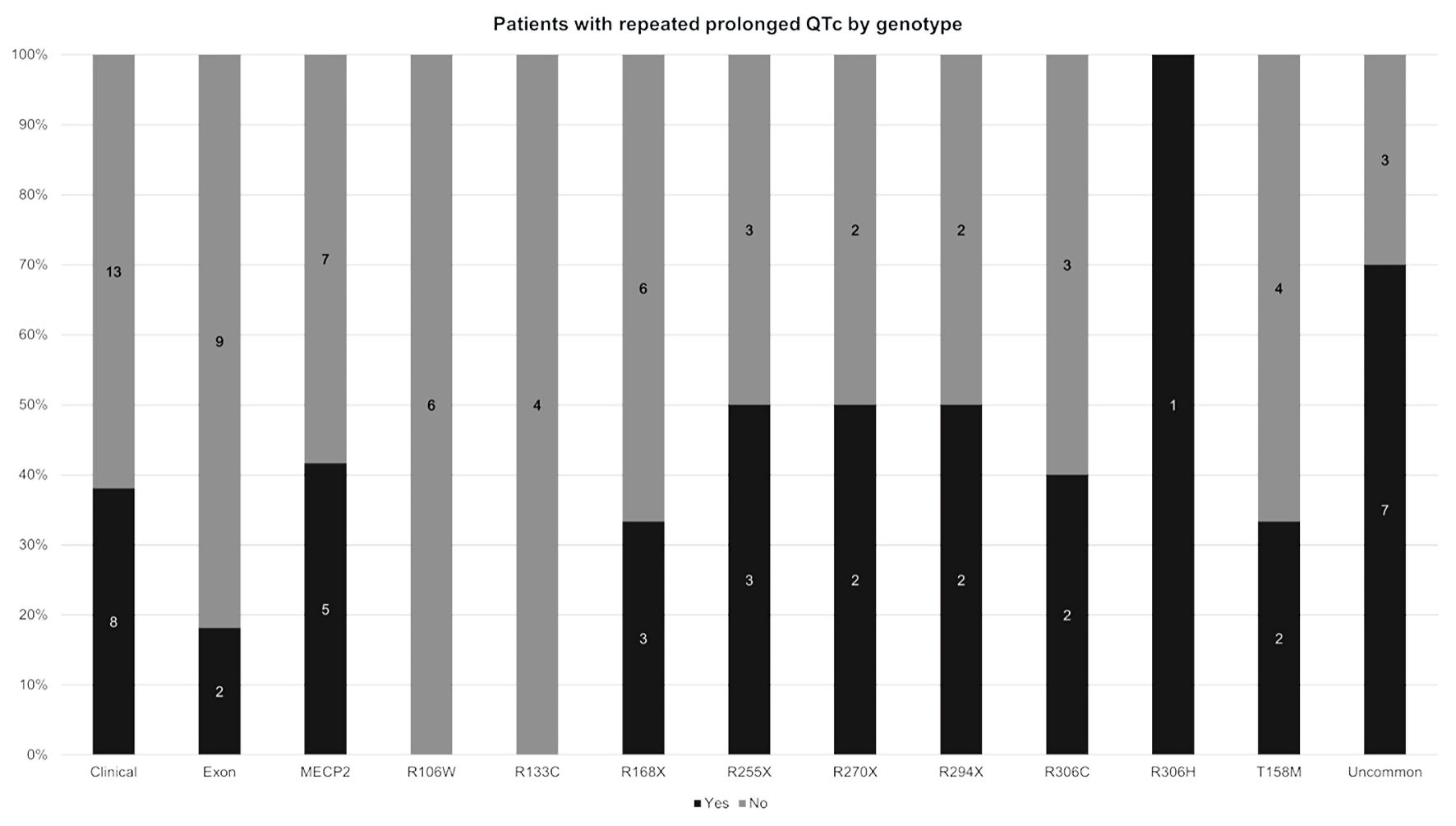 Click for large image | Figure 2. Distribution of patients with and without repeated prolonged QTc on ECG by RTT genotype. The black bar is the percentage of patients with repeated prolonged QTc on ECG. The gray bar is the percentage of patients without repeated prolonged QTc (i.e., patients with only one prolonged QTc on ECG or patients with no prolonged QTc on ECG). For each column, the percentage of patients with repeated prolonged QTc and the percentage of patients without repeated prolonged QTc add up to 100%. Clinical: clinical RTT as defined in the text; Uncommon: identified mutations besides R106W, R133c, R168X, R255X, R270X, R294x, R306C, R306H, T158M; QTc: corrected QT; ECG: electrocardiogram; RTT: Rett syndrome. |
T-wave parameters
The TpTe interval and the corrected JTp interval were longer in the RTT group but without reaching statistical significance. The MCS score was higher in the RTT group (97.8 (SD = 59) vs. 60.7 (SD = 24.6); P < 0.001). T waves in RTT patients were more flat, asymmetric, and had higher notch score when compared to the T waves of controls. PCA-2 percentages were higher in RTT patients than in controls (25.5 (SD = 13.2) vs. 17.3 (SD = 9.1); P < 0.001) (Table 2).
 Click to view | Table 2. Comparison of T wave Analysis Characteristics |
Sub-group comparisons of QTc and T-wave parameters
RTT mortality comparisons
The QTc was not statistically different between deceased RTT and living RTT patients. The JTp interval was longer in deceased patients (192 (SD = 36.7) ms vs. 206.3 (SD = 44) ms; P < 0.029). The T wave in deceased patients was significantly flatter, more asymmetric, and more notched. Therefore, the MCS score for deceased patients was higher compared to living patients (127.3 (SD = 75.7) vs. 94.8 (SD = 56.2); P = 0.001) (Table 3).
 Click to view | Table 3. QTc and T-wave Parameters by RTT Mortality |
Normal QTc group comparisons
RTT patients, who had normal QTc, still had significant differences in other T-wave parameters than normal QTc controls. In the RTT group, both JTp and TpTe lengthened significantly in long QT RTT patients when compared to normal QT RTT patients (Table 4).
 Click to view | Table 4. QTc and T-Wave Parameters Between Control With QTc < 460 ms and RTT With QTc < 460 ms |
RTT genotype comparisons
There was no statistically significant difference between the RTT genotypes on most of the ECG and T-wave parameters. Only the corrected JTp was significantly different across the three genotypes; the MECP2 group had the longest interval while the FOXG1 group had the shortest interval (Supplementary Material 1, www. cardiologyres.org).
| Discussion | ▴Top |
This study with a large, longitudinal, and genetically diverse group of RTT patients confirms that RTT patients exhibit longer QTc intervals than healthy controls. The prevalence of 50% of patients with at least one ECG with QT ≥ 460 ms is higher than previously reported [11]. However, this prolonged QT may reflect the background noise due to within-individual variation. Within-individual prolonged QT variation has been shown to be amplified when using Bazett’s formula possibly due to its “overcorrection” of the QT at higher HR such as seen in RTT patients [16]. On the other hand, 35% of patients had long QTc on two or more ECGs, which may reflect the true prevalence of prolonged QTc in RTT. Previously, certain MECP2 mutations have been associated with long QT (e.g., R255X, T158M) [10, 11]. We did not replicate that finding in our report. Among the common MECP2 mutations, the R270X and R294X groups had a higher proportion of repeated prolonged QTc on ECG patients than the R255X and T158M groups. However, overall, there was no statistical difference in the proportion of repeated prolonged QTc among the common MECP2 groups.
Persistently prolonged QTc, especially at 500 ms and above, has been associated with increased risk of malignant ventricular tachyarrhythmias [17]. However, ventricular tachycardia has only been documented in only one deceased patient with RTT. While this patient also had prolonged QTc, whether an episode of ventricular tachycardia preceded the demise and whether an arrhythmia was the direct cause the death could not be confirmed [8]. Moreover, most reported Holter data thus far have not shown frequent ventricular arrhythmias in RTT patients [6, 18, 19]. One deceased patient in our cohort did have long QTc on multiple ECGs, but the patient died due to acute respiratory failure. Since there is currently no accurate data on the incidence of sudden cardiac arrhythmic death in RTT, long-term and continuous cardiac monitoring with minimally invasive (e.g., implantable loop recorders) or wearable sensors may help further assess the relationship between long QT, arrhythmias, and outcomes.
Other components of the QT such as increased JTp and increased TpTe intervals have been identified as risk factors for sudden cardiac deaths in various populations [20, 21]. Increased TpTe is thought to represent increased heterogeneity of transmural cardiac repolarization that is associated with cardiac arrhythmogenicity [13]. In our study, both intervals were longer in RTT without reaching statistical significance. The observed long JTp is congruent with the persistence of late sodium channel current in mouse models of RTT [22, 23]. Interestingly, while deceased RTT patients had similar QTc and TpTe, they had significantly longer JTp than living RTT patients. The long JTp is driven mostly by the MECP2 and clinical groups, which account for all the mortalities. Measurements of JTp may be clinically more relevant in RTT.
This study further characterizes the qualitatively described abnormal T wave in RTT with quantitative scores [9]. These intuitive to visualize quantitative scores may not only guide the visual assessment of T-wave morphology but also help reduce the variability of the manual assessment of T-wave morphology especially in longitudinal measurements [24]. These scores have helped diagnosing long QT syndrome [15, 25]. Moreover, they have been independently associated with mortality in the general population [26]. These findings suggest the morphology of the T wave on ECG to be an important parameter to monitor in RTT patients. Since these scores can be implemented in ECG applications, they may help develop clinically meaningful risk-stratification criteria for sudden cardiac death in RTT. Finally, applications of other novel T-wave characterization methods may uncover other T-wave morphologies that are more sensitive for arrhythmic risk stratification [27].
In this study, we found that T waves are significantly more abnormal in RTT when compared to controls even when the QTc is normal. This finding suggests an intrinsic abnormal cardiac repolarization phenotype in RTT. This finding is important clinically since populations without long QT but with T-wave abnormalities that are similar to the abnormalities seen in congenital long QT syndrome appear to have increased arrhythmic risk. For example, in patients with bradycardia, those with T waves that resemble the T wave of congenital long QT syndrome type 2 are more at risk for developing ventricular tachycardia torsade de pointes. Future comparison with a matched pediatric cohort of long QT syndrome may explain the current perceived arrhythmic risk and further help risk stratify RTT patients.
RTT patients have sub-clinical myocardial dysfunction on echocardiograms [5]. T-wave abnormalities found in RTT may also reflect structural and mechanical abnormalities as seen in channelopathy and cardiomyopathy patients [28]. Repeated echocardiography with tissue Doppler/strain analysis and potentially cardiac magnetic resonance imaging (MRI) when the echocardiogram is abnormal may help further characterize the cardiac phenotype of RTT.
Limitations
This retrospective observational study was underpowered to perform mortality and genotype related analysis. The small number of patients in certain groups also precluded the analysis of the effects that physiologically significant interactions between groups (e.g., sex, mortality, and genotype) have on cardiac repolarizations. Moreover, the study lacked information on other confounding risk factors for abnormal cardiac repolarization such as concomitant medications, blood electrolytes, disease severity, epilepsy, or autonomic dysfunction. Serotonin reuptake inhibitors have been associated with long QT in RTT patients [11]. RTT patients frequently take antiepileptic drugs, some of which may be QT prolonging. On the other hand, sodium channel blocking agents have been shown to shorten QT in mice with MECP2 mutations [22, 23, 29]. Future studies evaluating the effects of antiepileptic drugs with sodium channel blocking properties and sodium channel blocker anti-arrhythmic medications on the abnormal cardiac repolarization of RTT patients should be pursued. Sudden death with epilepsy may occur in RTT. However, it is challenging to determine what is and is not a seizure in RTT patients. It would also be challenging to study the role of epilepsy in sudden death in RTT patients due its repetitive remission and relapsing nature over the lifespan of RTT patients. Finally, RTT patients’ autonomic dysfunction (e.g., reduced HR variability), has strongly been implicated as the major cause of sudden death in RTT [30]. Reduced HR variability reflecting the increased sympathetic activation and/or decrease in parasympathetic activation has been associated with prolonged QTc in RTT patients. Reduced HR variability has also been associated with increased cardiac repolarization heterogeneity and cardiac arrhythmias in many different populations. Future studies investigating the effects of changes in HR variability such as during natural circadian oscillation or when provoked (e.g., change in body position, cardiac pacing, or medications) have on QT duration and other parameters of cardiac repolarization are needed to elucidate the pathophysiology of autonomic dysfunction vis-a-vis cardiac repolarization and cardiac arrhythmic death in RTT.
Conclusions
RTT patients have abnormal cardiac repolarization including long QTc, increased heterogeneity of cardiac repolarization, and abnormal T-wave morphology. Further longitudinal characterizations of these markers may help in better understanding of the RTT cardiac electrophysiologic phenotypes leading to helpful risk stratification for malignant cardiac arrhythmias of these patients.
| Supplementary Material | ▴Top |
Suppl 1. QTc and T-wave parameters by genotype.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
Each author declares that they do not have any competing interests pertaining to this study.
Author Contributions
All authors planned, conceived and designed the study. MPC and HHN acquired the data. HHN analyzed the data. MPC, MCJ, WAS, and HHN interpreted the electrophysiology data. RCR, JLW, PTH, CMB, DMMA, and HMK interpreted the clinical and genetic data. MPC wrote the first draft of the manuscript. All authors critically reviewed and edited the manuscript. All authors agreed to the submission of the manuscript.
Data Availability
The dataset analyzed during the current study is available from the corresponding author on reasonable request.
Abbreviations
RTT: Rett syndrome; ECG: electrocardiogram; PCA: principal component analysis; MCS: morphology combination score
| References | ▴Top |
- Fu C, Armstrong D, Marsh E, Lieberman D, Motil K, Witt R, Standridge S, et al. Consensus guidelines on managing Rett syndrome across the lifespan. BMJ Paediatr Open. 2020;4(1):e000717.
doi pubmed pmc - Olson HE, Demarest ST, Pestana-Knight EM, Swanson LC, Iqbal S, Lal D, Leonard H, et al. Cyclin-dependent kinase-like 5 deficiency disorder: clinical review. Pediatr Neurol. 2019;97:18-25.
doi pubmed pmc - Mitter D, Pringsheim M, Kaulisch M, Plumacher KS, Schroder S, Warthemann R, Abou Jamra R, et al. FOXG1 syndrome: genotype-phenotype association in 83 patients with FOXG1 variants. Genet Med. 2018;20(1):98-108.
doi pubmed - Tarquinio DC, Hou W, Neul JL, Kaufmann WE, Glaze DG, Motil KJ, Skinner SA, et al. The changing face of survival in Rett syndrome and MECP2-related disorders. Pediatr Neurol. 2015;53(5):402-411.
doi pubmed pmc - De Felice C, Maffei S, Signorini C, Leoncini S, Lunghetti S, Valacchi G, D'Esposito M, et al. Subclinical myocardial dysfunction in Rett syndrome. Eur Heart J Cardiovasc Imaging. 2012;13(4):339-345.
doi pubmed - Guideri F, Acampa M, DiPerri T, Zappella M, Hayek Y. Progressive cardiac dysautonomia observed in patients affected by classic Rett syndrome and not in the preserved speech variant. J Child Neurol. 2001;16(5):370-373.
doi pubmed - Shioda T, Takahashi S, Kaname T, Yamauchi T, Fukuoka T. MECP2 mutation in a boy with severe apnea and sick sinus syndrome. Brain Dev. 2018;40(8):714-718.
doi pubmed - Guideri F, Acampa M. Sudden death and cardiac arrhythmias in Rett syndrome. Pediatr Cardiol. 2005;26(1):111.
doi pubmed - Sekul EA, Moak JP, Schultz RJ, Glaze DG, Dunn JK, Percy AK. Electrocardiographic findings in Rett syndrome: an explanation for sudden death? J Pediatr. 1994;125(1):80-82.
doi pubmed - Crosson J, Srivastava S, Bibat GM, Gupta S, Kantipuly A, Smith-Hicks C, Myers SM, et al. Evaluation of QTc in Rett syndrome: Correlation with age, severity, and genotype. Am J Med Genet A. 2017;173(6):1495-1501.
doi pubmed pmc - Clark BC, Kopp A, Morey W, Djukic A. Serial follow-up of corrected QT interval in Rett syndrome. Dev Med Child Neurol. 2020;62(7):833-836.
doi pubmed - Viskin S, Chorin E, Viskin D, Hochstadt A, Schwartz AL, Rosso R. Polymorphic ventricular tachycardia: terminology, mechanism, diagnosis, and emergency therapy. Circulation. 2021;144(10):823-839.
doi pubmed - Prenner SB, Shah SJ, Goldberger JJ, Sauer AJ. Repolarization heterogeneity: beyond the QT interval. J Am Heart Assoc. 2016;5(5):e003607.
doi pubmed pmc - Jutzy GJ, Heydemann P, Buhrfiend C, Nguyen HH. Abstract 16769: QT prolongation and variability among patients with rett syndrome. Circulation. 2018;138(Suppl_1):A16769-A16769.
- Porta-Sanchez A, Spillane DR, Harris L, Xue J, Dorsey P, Care M, Chauhan V, et al. T-wave morphology analysis in congenital long QT syndrome discriminates patients from healthy individuals. JACC Clin Electrophysiol. 2017;3(4):374-381.
doi pubmed - Gueta I, Klempfner R, Markovits N, Halkin H, Segev S, Rott D, Peled Y, et al. Clinically significant incidental QTc prolongation is subject to within-individual variability. Ann Noninvasive Electrocardiol. 2020;25(2):e12699.
doi pubmed pmc - Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, Vicentini A, et al. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348(19):1866-1874.
doi pubmed - Guideri F, Acampa M, Hayek G, Zappella M, Di Perri T. Reduced heart rate variability in patients affected with Rett syndrome. A possible explanation for sudden death. Neuropediatrics. 1999;30(3):146-148.
doi pubmed - Carroll MS, Ramirez JM, Weese-Mayer DE. Diurnal variation in autonomic regulation among patients with genotyped Rett syndrome. J Med Genet. 2020;57(11):786-793.
doi pubmed pmc - Panikkath R, Reinier K, Uy-Evanado A, Teodorescu C, Hattenhauer J, Mariani R, Gunson K, et al. Prolonged Tpeak-to-tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol. 2011;4(4):441-447.
doi pubmed pmc - O'Neal WT, Singleton MJ, Roberts JD, Tereshchenko LG, Sotoodehnia N, Chen LY, Marcus GM, et al. Association between QT-interval components and sudden cardiac death: the ARIC Study (Atherosclerosis Risk in Communities). Circ Arrhythm Electrophysiol. 2017;10(10):e005485.
doi pubmed pmc - McCauley MD, Wang T, Mike E, Herrera J, Beavers DL, Huang TW, Ward CS, et al. Pathogenesis of lethal cardiac arrhythmias in Mecp2 mutant mice: implication for therapy in Rett syndrome. Sci Transl Med. 2011;3(113):113ra125.
doi pubmed pmc - Cheng H, Charles I, James AF, Abdala AP, Hancox JC. Delayed ventricular repolarization and sodium channel current modification in a mouse model of Rett syndrome. Int J Mol Sci. 2022;23(10):5735.
doi pubmed pmc - Hnatkova K, Malik M. Sources of QTc variability: Implications for effective ECG monitoring in clinical practice. Ann Noninvasive Electrocardiol. 2020;25(2):e12730.
doi pubmed pmc - Hermans BJM, Stoks J, Bennis FC, Vink AS, Garde A, Wilde AAM, Pison L, et al. Support vector machine-based assessment of the T-wave morphology improves long QT syndrome diagnosis. Europace. 2018;20(suppl_3):iii113-iii119.
doi pubmed - Isaksen JL, Ghouse J, Graff C, Olesen MS, Holst AG, Pietersen A, Nielsen JB, et al. Electrocardiographic T-wave morphology and risk of mortality. Int J Cardiol. 2021;328:199-205.
doi pubmed - Hermans BJM, Bennis FC, Vink AS, Koopsen T, Lyon A, Wilde AAM, Nuyens D, et al. Improving long QT syndrome diagnosis by a polynomial-based T-wave morphology characterization. Heart Rhythm. 2020;17(5 Pt A):752-758.
doi pubmed - Sauer AJ, Selvaraj S, Aguilar FG, Martinez EE, Wilcox JE, Passman R, Goldberger JJ, et al. Relationship between repolarization heterogeneity and abnormal myocardial mechanics. Int J Cardiol. 2014;172(1):289-291.
doi pubmed pmc - Herrera JA, Ward CS, Pitcher MR, Percy AK, Skinner S, Kaufmann WE, Glaze DG, et al. Treatment of cardiac arrhythmias in a mouse model of Rett syndrome with Na+-channel-blocking antiepileptic drugs. Dis Model Mech. 2015;8(4):363-371.
doi pubmed pmc - Singh J, Lanzarini E, Santosh P. Autonomic dysfunction and sudden death in patients with Rett syndrome: a systematic review. J Psychiatry Neurosci. 2020;45(3):150-181.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


