| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 15, Number 5, October 2024, pages 358-368
Evaluating the Prognostic Value of the Modified H2FPEF Score in Patients With Heart Failure With Preserved Ejection Fraction
Ya Qiong Jina, Lu Genga, Yue Lia, Ke Ke Wanga, Bing Xiaoa, Meng Xiao Wanga, Xue Ying Gaoa, Jie Zhanga, Xiu Chun Yanga, Jing Chao Lua, b
aDepartment of Cardiology, The Second Hospital of Hebei Medical University, Shijiazhuang City, Hebei 050000, China
bCorresponding Author: Jing Chao Lu, Department of Cardiology, The Second Hospital of Hebei Medical University, Shijiazhuang City, Hebei 050000, China
Manuscript submitted March 13, 2024, accepted August 19, 2024, published online September 16, 2024
Short title: Modified H2FPEF Score in HFpEF Patients
doi: https://doi.org/10.14740/cr1635
| Abstract | ▴Top |
Background: The H2FPEF score, a convenient tool developed for diagnosing heart failure with preserved ejection fraction (HFpEF), exhibited useful prognostic utility in HFpEF. However, the applicability and the prognostic value of the H2FPEF score in Chinese HFpEF patients have yet to be fully confirmed. The study aimed to evaluate the effect of modified H2FPEF score on the prognosis of Chinese HFpEF patients.
Methods: In this retrospective study, we calculated the H2FPEF scores by body mass index (BMI) ≥ 25 kg/m2 and 30 kg/m2 respectively, for 497 consecutive HFpEF patients in China. Subjects were divided into low- (0 - 3 points), intermediate- (4 - 6 points), and high-score (7 - 9 points) groups. The primary and secondary endpoints were heart failure (HF)-related events and acute coronary syndrome (ACS), respectively. Cox proportional hazard models were applied to calculate hazard ratios (HRs). Receiver operating characteristic (ROC) curves and areas under the curve (AUC) were used to evaluate the prediction of the H2FPEF score for adverse outcomes.
Results: Over a mean follow-up of 40.46 ± 6.52 months, the primary and secondary endpoints occurred in 168 patients (33.8%) and 97 patients (19.5%), respectively. By the definition of obesity as BMI ≥ 25 kg/m2, a higher incidence of HF-related events and ACS was observed among those with a higher modified H2FPEF score. The modified H2FPEF significantly predicted HF-related events (AUC: 0.723; 95% confidence interval (CI): 0.676 - 0.770; P < 0.001) and ACS (AUC: 0.670; 95% CI: 0.608 - 0.731; P < 0.014) with higher power than the H2FPEF score calculated by BMI ≥ 30 kg/m2. The cutoff of the modified H2FPEF score was 6.5 for detecting HF-related events and ACS.
Conclusions: The modified H2FPEF score, using BMI ≥ 25 kg/m2 to define obesity, could more effectively predict the occurrence of subsequent cardiovascular events in Chinese HFpEF patients. The modified H2FPEF score above 6.5 is a risk factor for adverse cardiovascular events in HFpEF patients.
Keywords: Cardiovascular events; Heart failure with preserved ejection fraction; Heart failure; Acute coronary syndrome; Risk stratification
| Introduction | ▴Top |
Heart failure with preserved ejection fraction (HFpEF), characterized by pathological increases in cardiac filling pressures at rest or with exertion, is a common clinical syndrome associated with high morbidity and mortality rates [1, 2]. HFpEF has progressed to be the dominant form of heart failure (HF) worldwide, accounting for approximately 50% of all hospital admissions for HF [3-5]. Meanwhile, the evidence suggests the prevalence of HFpEF is increasing at a rate of 1% per year [6-8]. Although HFpEF was historically called diastolic HF, it is now widely recognized that HFpEF is a systemic syndrome involving multiple pathophysiological abnormalities beyond left ventricular diastolic dysfunction [9-13]. Symptoms of HFpEF are non-specific, and diagnosis might be elusive, resulting in a diagnostic and therapeutic challenge. Exercise right heart catheterization is the current gold standard test for diagnosing HFpEF when HFpEF is suspected but left ventricular filling pressures at rest are normal. Nevertheless, exercising during right heart catheterization is not universally available [14, 15]. Under these circumstances, there was a great emphasis on finding simple and noninvasive indices of HFpEF diagnosis.
The H2FPEF score, a weighted score based on six variables that ranged from 0 to 9, was proposed by Reddi et al to diagnose symptomatic euvolemic patients as HFpEF in 2018 [16]. In the H2FPEF score system, the set of predictive variables includes obesity, atrial fibrillation (AF), age > 60 years, treatment with ≥ 2 antihypertensives, echocardiographic E/e’ ratio > 9, and echocardiographic pulmonary artery systolic pressure (PAP) > 35 mm Hg [16]. The H2FPEF score has been validated as a practical diagnostic tool for HFpEF [17, 18]. Furthermore, recent evidence supported that the H2FPEF score was also a significant predictor for adverse cardiovascular events in HFpEF patients [19, 20]. Although several studies have tested the prognostic value of the H2FPEF score among HFpEF patients, whether the results could be widely applicable in Chinese HFpEF patients is not well validated. In this retrospective study, we aimed to examine the applicability of the H2FPEF score and the predictive value of the H2FPEF score among Chinese HFpEF patients in our institution.
| Materials and Methods | ▴Top |
Study subjects
We retrospectively assessed the HFpEF patients hospitalized at the Second Hospital of Hebei Medical University from January 1, 2018 to June 31, 2020. The diagnostic criteria were clinical symptoms or signs of HF with a left ventricular ejection fraction (LVEF) ≥ 50%, and elevated levels of natriuretic peptides (B-type natriuretic peptide (BNP) > 35 ng/L or N-terminal pro B-type natriuretic peptide (NT-proBNP) > 125 ng/L); and at least one of the following additional criteria: 1) relevant structural heart disease, including left ventricular (LV) hypertrophy and/or left atrial enlargement (left atrial volume index > 34 mL/m2, left ventricular mass index (LVMI) ≥ 115 g/m2 in men or ≥ 95 g/m2 in women); 2) diastolic dysfunction (E/e’ ratio ≥ 13 and/or e’ < 9 cm/s). The exclusion criteria included: 1) acute myocardial infarction (MI) within 1 month on admission; 2) congenital heart disease; 3) primary cardiomyopathy; 4) severe valvular disease, 5) end-stage renal failure (estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2); 6) acute exacerbation of the chronic obstructive pulmonary disease. A total of 553 consecutive patients (> 18 years old) with HFpEF were hospitalized at our institution between January 1, 2018 and June 31, 2020; 56 patients were eliminated due to incomplete data during the follow-up phase. Finally, the remaining 497 HFpEF patients were enrolled in this study. All subjects provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of the Second Hospital of Hebei Medical University (approval number 2022-R677).
H2FPEF score
Body mass index (BMI), treatment with ≥ 2 antihypertensives, presence of AF, PAP (> 35 mm Hg), age (> 60 years), and echocardiographic E/e’ ratio (> 9) were all used to calculate the H2FPEF score for each patient. In terms of the typical body shape of the Asian population, according to previous studies and the World Health Organization Asian classification suggested as obesity [21], we analyzed the data by defining obesity as BMI ≥ 30 kg/m2 and ≥ 25 kg/m2, respectively. The H2FPEF score was available in all 497 patients with HFpEF. The study population was divided into three groups according to the H2FPEF score to evaluate the clinical features and echocardiographic parameters: the low-score group (0 - 3 points), the intermediate-score group (4 - 6 points), and the high-score group (7 - 9 points). Figure 1 depicts the flow chart for the investigation.
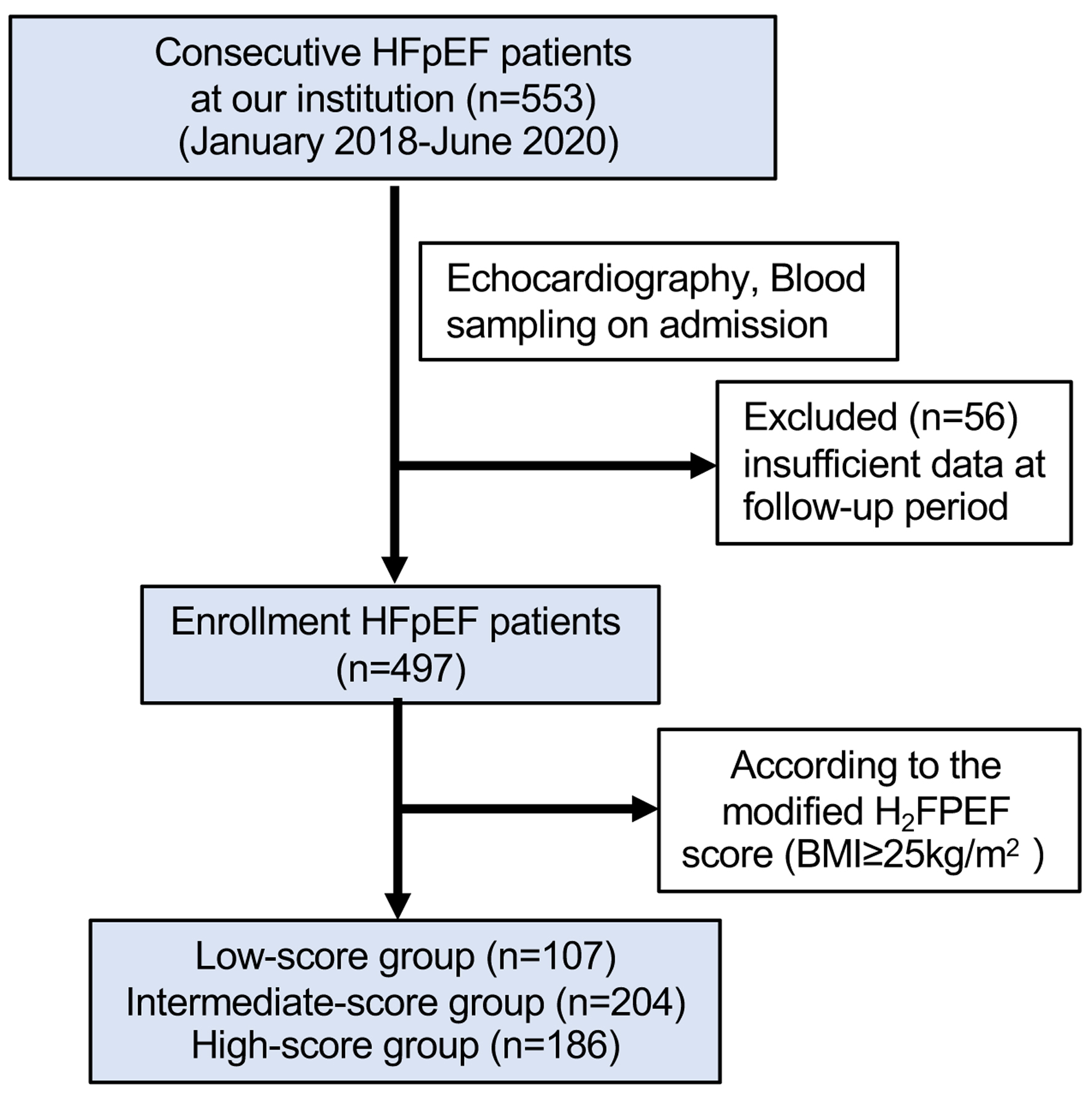 Click for large image | Figure 1. The flow chart for the investigation. HFpEF: heart failure with preserved ejection fraction. |
Clinical variables and echocardiography
The baseline demographic data, clinical characteristics, comorbidities, drug and intervention therapy, laboratory values, arrhythmias, and echocardiography findings were collected and recorded. Hypertension was defined as a recorded blood pressure ≥ 140/90 mm Hg or taking antihypertensive medications, as previously described. Diabetes was defined as a fasting blood glucose level of ≥ 126 mg/dL or current treatment for diabetes. Smoking status, including past and current smoking, was determined via an interview. Current smoker was defined as smoking status on admission.
Subjects were required to fast over 8 h before venous blood collection, and blood samples were usually obtained in the morning after admission. The BNP or NT-proBNP levels were analyzed by a commercially available assay (Abbott Japan, Matsudo, Japan) in the hospital clinical laboratory. The eGFR was calculated using the Japanese Society of Nephrology formula [22].
All subjects underwent echocardiography by experienced cardiac sonographers who were blind to this study. As described previously, LVEF was measured by modified Simpson’s method. The ratio of early transmittal flow velocity to early diastolic mitral annular velocity (E/e’) was assessed by tissue Doppler, and LVMI was measured by echocardiography (Philips IE33 system, Andover, MA, USA; Philips EPIQ 7C system, Andover, MA, USA) [23].
Follow-up and endpoints
The observations were made by investigators who were blind to this study. The agreements for assessing outcomes were performed under the consensus of multiple evaluators. Patients were followed up at our outpatient clinics until June 31, 2021, or until an endpoint occurred. The primary and secondary endpoints were HF-related events (cardiovascular death and hospitalization for HF decompensation) and acute coronary syndrome (ACS) (nonfatal MI and unstable angina pectoris) at 42 months, respectively. Cardiovascular death was defined as death due to MI, congestive heart failure, or documented sudden death without non-cardiovascular causes. Hospitalization for HF decompensation was diagnosed if the patient had typical HF symptoms or objective signs of worsening HF that required intravenous drug administration when admission. ACS is caused by a critical obstruction of a coronary artery because of atherosclerotic coronary artery disease. Three specific conditions are included: ST-elevation MI (STEMI), non-ST elevation MI (NSTEMI), and unstable angina. Cardiovascular events were ascertained from a review of the medical records and confirmed by direct contact with the patients, their families, and their physicians. For patients with more than one cardiovascular event, only the first event was counted as an event, except for the cardiovascular death.
Statistical analysis
Constant values were expressed as means ± standard deviations (x ± SDs), whereas non-normally distributed data were expressed as the median (interquartile range). Differences between the three groups were assessed by one-way analysis of variance (ANOVA) or the Kruskal-Wallis test for continuous variables and the Chi-squared test for categorical variables. Fisher’s exact probability method was used for counting data with smaller sample sizes. The cumulative incidence and differences between groups of HF-related events or ACS events were determined by a Kaplan-Meier curve and the log-rank test, respectively. Univariate Cox proportional hazard regression was used to identify significant predictors of the outcomes. Significant predictors were then entered into multivariate analysis and variables that will cause internal correlations were excluded. The proportional hazards assumption was examined by using R. When the proportional hazards assumption does not hold, Cox regression models with time-varying coefficient were used. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. The factors of the BMI, prevalence of hypertension, prevalence of AF, age, and Doppler echocardiographic E/e’ ratio were components of the H2FPEF score, and we thought these variables caused internal correlations with the H2FPEF score variable. Receiver operating characteristic (ROC) curves were constructed, and the areas under the curve (AUC) were calculated for the H2FPEF score to predict future HF-related events and ACS events. We defined the cutoff value of the H2FPEF score by utilizing the sensitivity, specificity, and likelihood ratio for future HF-related events and ACS events.
Satistical analyses were analyzed by the software Statistical Package for Social Sciences (SPSS) Version 24.0 (IBM Japan, Tokyo, Japan) and R program (Version 4.1.1 GUI 1.77; High Sierra build). A P-value < 0.05 was considered statistically significant.
| Results | ▴Top |
A total number of 497 patients with HFpEF were enrolled in this study. Overall, the patients had a mean age of 69.05 ± 10.47 years, and 57.9% were female. The mean systolic and diastolic blood pressures were 135.30 ± 20.78 and 79.02 ± 13.07 mm Hg, respectively (Table 1). Over a mean follow-up of 40.46 ± 6.52 months, the primary endpoint occurred in 168 patients (33.8%), among which 31 patients (6.2%) died of cardiovascular diseases and 148 patients (29.8%) were re-hospitalized due to heart failure. The secondary endpoint occurred in 97 patients (19.5%) (Table 2).
 Click to view | Table 1. Baseline Characteristics of HFpEF Patients According to the Modified H2FPEF Score |
 Click to view | Table 2. Cardiovascular Events in the Study Groups According to the Modified H2FPEF Score |
H2FPEF score calculated by BMI ≥ 30 kg/m2
The average H2FPEF score was 4.59 ± 2.03 when calculated by BMI ≥ 30 kg/m2. The patient numbers (percentage) of low- (1 - 3 points), intermediate- (4 - 6 points), and high-score (7 - 9 points) were 167 (33.6%), 230 (46.3%), and 100 (20.1%), respectively (Supplementary Tables 1 and 2, www.cardiologyres.org). We found significantly higher rates of primary and secondary points in high-score group patients than those in the low-score group (P < 0.05) (Supplementary Table 3, www.cardiologyres.org). The Cox proportional hazards analysis indicated that the H2FPEF score was associated with the primary point (HR: 1.065; 95% CI: 1.019 - 1.112; P < 0.001) but not the secondary point (HR: 1.054.370; 95% CI: 0.997 - 1.115; P = 0.066) (Supplementary Table 4, www.cardiologyres.org). ROC analysis showed the H2FPEF score had a predictive role for HF-related events (AUC: 0.666, 95% CI: 0.616 - 0.716; P < 0.001) and ACS (AUC: 0.626, 95% CI: 0.563 - 0.689; P < 0.001 (Supplementary Figure 1, www.cardiologyres.org).
H2FPEF score calculated by BMI ≥ 25 kg/m2
Clinical characteristics
According to the modified H2FPEF score calculated by BMI ≥ 25 kg/m2, the patient numbers (percentage) of low- (1 - 3 points), intermediate- (4 - 6 points), and high-score (7 - 9 points) were 107 (21.5%), 204 (41.0%), and 186 (37.4%), respectively (Table 1 and Supplementary Table 5, www.cardiologyres.org).
As compared to the low-score group, patients in the high-score group showed elevated age, BMI, heart rate, serum creatinine, uric acid (UA), hemoglobin levels, and declined eGFR, with higher prevalence of hypertension, AF and previous hospitalization for HF. Regarding the resting echocardiography, a higher E/e’ ratio and PAP were observed in high-score group patients, with a larger left atrial diameter (LAD) and right atrial diameter (RAD). Meanwhile, compared to the patients with low H2FPEF scores, patients with high H2FPEF scores received more spironolactone, loop diuretic and anticoagulant drugs (Table 1).
Over the follow-up period, patients in the high-score group had higher rates of re-hospitalization (P < 0.001) and ACS events (P < 0.001) than those in the low-score group (Table 2, Supplementary Tables 6 and 7, www.cardiologyres.org).
Kaplan-Meier curves
The Kaplan-Meier analysis stratified by the modified H2FPEF score showed a significantly higher probability of primary endpoint in the patients with a high H2FPEF score at follow-up (P < 0.001) (Fig. 2a). Additionally, the survival analysis indicated that the modified H2FPEF score successfully stratified the patients for HF re-hospitalization (Supplementary Figure 2B, www.cardiologyres.org, log-rank test P < 0.001) but not for cardiovascular death (Supplementary Figure 2A, www.cardiologyres.org, log-rank test P = 0.177). A significantly higher probability of the secondary endpoint was also observed in the high-score group (P < 0.001) (Fig. 2b).
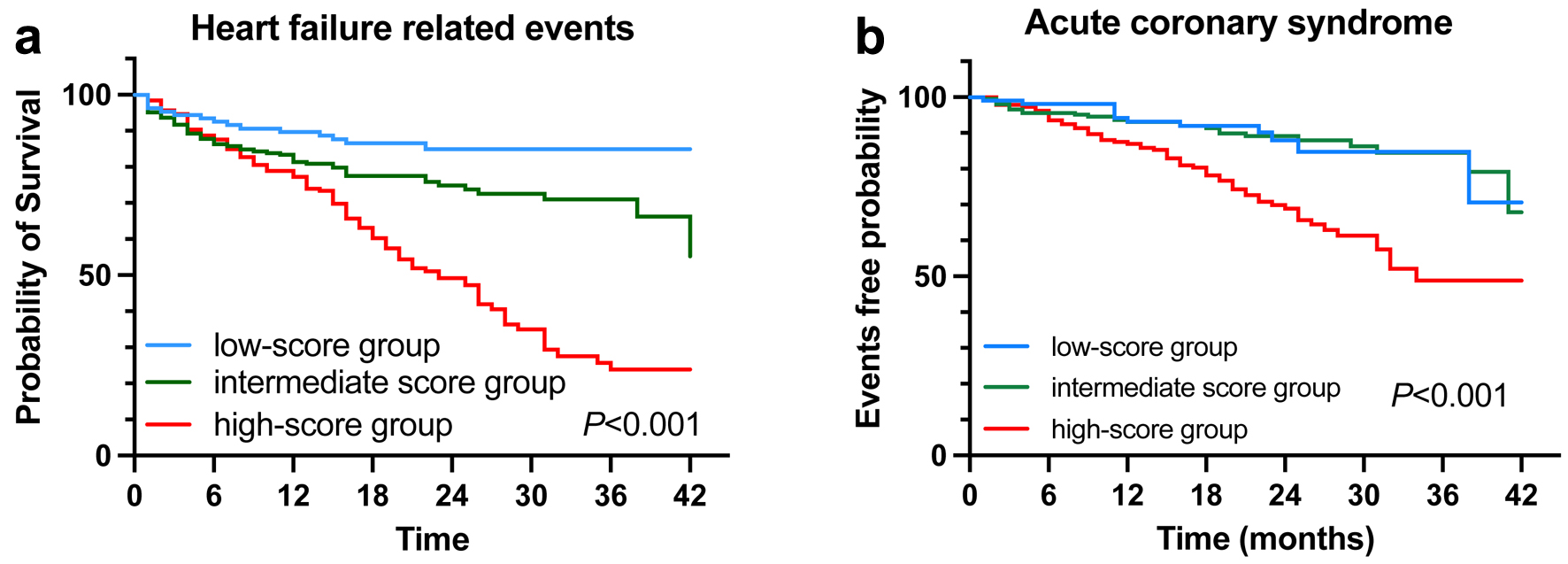 Click for large image | Figure 2. Kaplan-Meier analyses for primary (a) and secondary (b) endpoints according to the modified H2FPEF scores by BMI ≥ 25 kg/m2. BMI: body mass index. |
Cox proportional hazards analysis
Table 3 shows the modified H2FPEF score was a significant predictor of HF-related events (HR: 1.085; 95% CI: 1.038 - 1.134; P < 0.001) and ACS events (HR: 1.072; 95% CI: 1.016 - 1.371; P = 0.011). In addition, digoxin (HR: 2.383; 95% CI: 1.212 - 5.066; P = 0.024) was independently associated with a higher risk of HF-related events.
 Click to view | Table 3. Cox Proportional Hazards Analysis of Cardiovascular Events in HFpEF Patients |
ROC analysis
As shown in Figure 3, the AUC of the modified H2FPEF score for detecting HF-related events and ACS were 0.723 (95% CI: 0.676 - 0.770; P < 0.001) and 0.670 (95% CI: 0.608 - 0.731; P < 0.001). The modified H2FPEF score exhibited higher power compared to the H2FPEF score calculated by BMI ≥ 30 kg/m2. Using the cutoff value of 6.5, the sensitivity and specificity of the modified H2FPEF score for detecting HF-related events were 60.2% and 74.5%, respectively. The cutoff value of the modified H2FPEF score for the detection of ACS was 6.5 with 61.9% of sensitivity and 68.5% of specificity (Supplementary Tables 8 and 9, www.cardiologyres.org).
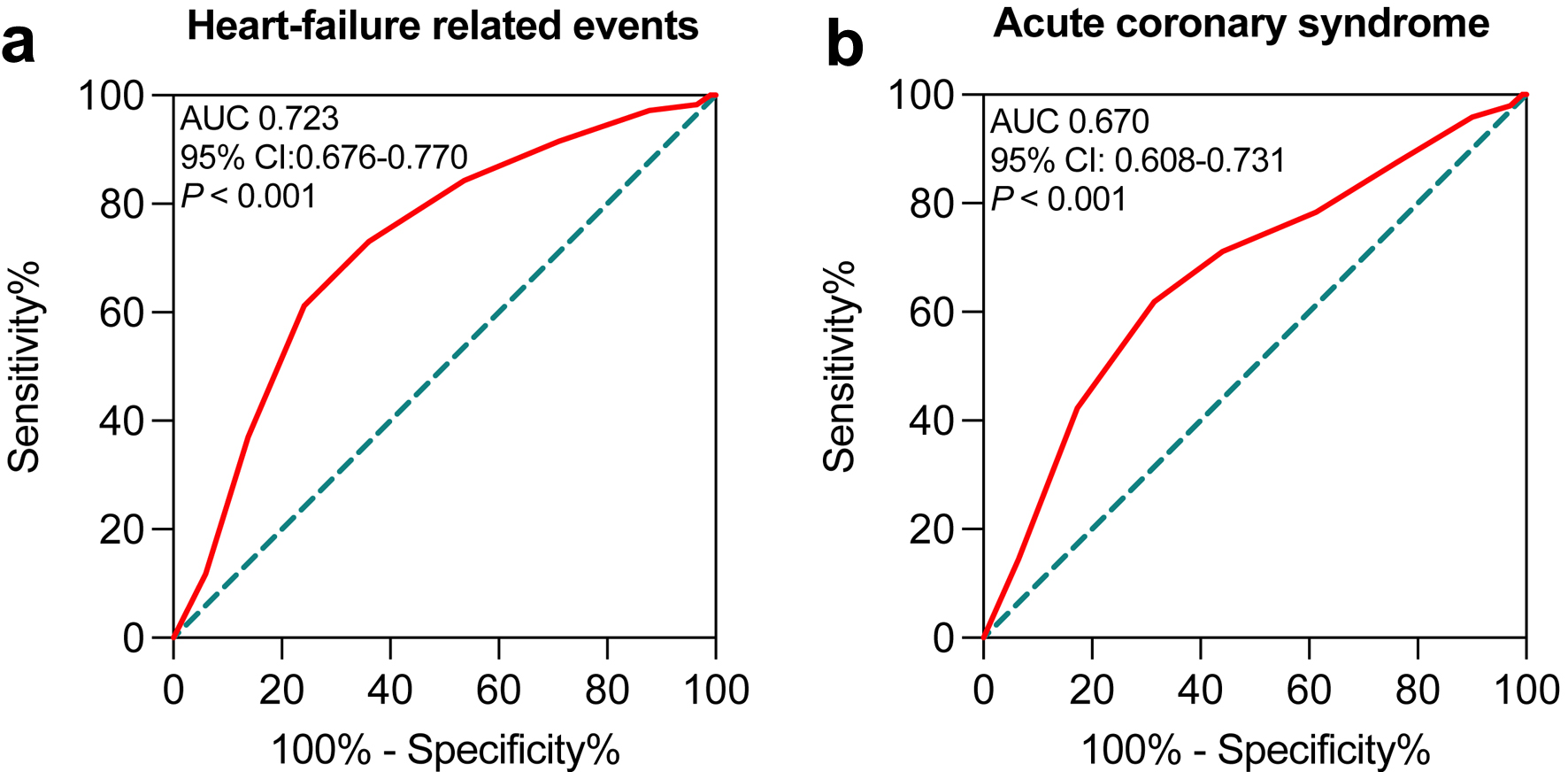 Click for large image | Figure 3. ROC curves for the modified H2FPEF scores by BMI ≥ 25 kg/m2 to predict primary (a) and secondary (b) endpoints in HFpEF patients. BMI: body mass index; HFpEF: heart failure with preserved ejection fraction; ROC: receiver operating characteristic. |
| Discussion | ▴Top |
In this study, we retrospectively assessed the predictive value of the H2FPEF score among Chinese HFpEF patients. When obesity was defined by BMI ≥ 25 kg/m2, HFpEF patients with high modified H2FPEF scores had a significantly higher probability of HF-related events and ACS. The modified H2FPEF score was an independent predictor of future HF-related events and ACS with higher power than the H2FPEF score calculated by BMI ≥ 30 kg/m2. And the optimum cut-off value of H2FPEF was 6.5. In conclusion, the modified H2FPEF score was a predictive algorithm for adverse cardiovascular events in Chinese HFpEF patients.
Over the past three decades, the prevalence of HFpEF relative to total HF prevalence rose from 41% to 56%. Simultaneously, the prevalence of heart failure with reduced ejection fraction (HFrEF) and heart failure with midrange ejection fraction fell from 44% to 31% and 15% to 13%, respectively [5, 24]. This trend may have been influenced by increased awareness of HFpEF in recent decades [25]. The aging population also contributes significantly to the increase in the number and proportion of HFpEF patients. Concomitant risk factors in modern life, such as obesity, diabetes mellitus, and chronic kidney disease, are partly responsible for the rise of HFpEF prevalence. The high frequency of comorbidities not only raises the chance of hospitalization and death but also leads to symptomatic deterioration, which results in decreased quality of life and functional capacity in patients. Early identification of at-risk patients and aggressive management are critical to preventing HFpEF and its progression.
The H2FPEF score, including six variables, is a practical diagnostic tool and a prognostic algorithm for HFpEF. Obesity, a common risk factor for cardiovascular diseases, is defined as BMI ≥ 30 kg/m2 in the initial H2FPEF score [16-18]. However, there is a debate regarding the best BMI classification for the Asian population because of their structural variations compared to the western population [21, 26]. In 2016, the Global BMI Mortality Collaboration conducted a meta-analysis of 239 prospective studies that showed that all-cause mortality in East Asians increased significantly with a BMI ≥ 25 kg/m2 [27]. Therefore, different BMI criteria for obesity are needed for different races. Based on the fact that Asians have higher morbidity and mortality even with lower BMI and waist circumference (WC), the WHO Asia-Pacific region defined BMI ≥ 23 kg/m2 as overweight and ≥ 25 kg/m2 as obese [28]. Recently, some studies among the Asian population used a different cutoff of BMI to examine the role of H2FPEF score in HFpEF prognosis. Defined obesity as BMI ≥ 25 kg/m2, Sueta et al reported that the H2FPEF score was a potentially useful marker for predicting cardiovascular and HF-related events in Japanese HFpEF patients [17]. Nevertheless, Tao et al indicated that the H2FPEF score calculated by BMI ≥ 30 kg/m2 had excellent predictive value for 1-year rehospitalization in Chinese HfpEF patients [29]. In this retrospective study, we found that the H2FPEF score with BMI ≥ 25 kg/m2 exhibited a higher power for predicting adverse outcomes in Chinese HfpEF patients. Although the results needed to be evaluated in larger study cohorts, our findings highlighted the need for the application of population-specific and culturally appropriate metrics when assessing cardiovascular health.
In our study cohort, the Kaplan-Meier analysis indicated that a significantly higher probability of HF-related events was observed in the high-score group. However, the H2FPEF score was not correlated with cardiovascular mortality in the last episode of the follow-up. This may be due to the small sample size and relatively short follow-up period.
The Cox proportional hazards analysis showed that in addition to the modified H2FPEF score, digoxin treatment was related to adverse HF outcomes in HFpEF. Although numerous clinical studies have found that HF patients treated with digoxin may have a higher risk with poor outcomes, digoxin therapy in HF remained controversial [30-32]. A multicenter study conducted among old patients (> 70 years) with HFpEF showed that digoxin treatment was associated with increased mortality and re-admission, particularly in those with lower heart rates [31]. However, a recent study reported that digoxin initiation prior to hospital discharge did not affect 30-day or 6-year outcomes in old hospitalized patients with HFpEF [33]. Another score-matched cohort of hospitalized HFpEF patients with AF who were not on digoxin at the time of hospitalization found that initiation of digoxin before hospital discharge was associated with a lower risk of HF re-admission but had no effect on mortality rate [34]. A potential explanation for the inconsistent results may lie in the study design and bias associated with the “time-dependent” initiation of digoxin. Patients may benefit from the early hemodynamic effects of digoxin. However, prevalent digoxin use is a marker of disease severity and is usually associated with a higher risk of adverse outcomes [35, 36].
The available data indicated that a high modified H2FPEF score was associated with ACS events in HFpEF patients. Although pretty common in HFpEF patients, the interaction of ACS and HFpEF has been underestimated [37, 38]. Compared to patients with only HFpEF, a more significant deterioration of LV function and a worse prognosis were observed in patients with HFpEF and coronary artery disease [39, 40]. Ischemic heart disease conferred an approximate 20% increase in the risk of major adverse renal and cardiovascular events for patients with HFpEF. On the other hand, a history of MI could be significantly linked to the risk of HFpEF. The prevalence of new-onset HF was reported to be 9.5% in ACS patients during a median follow-up of 63 months [41]. One of the possible explanations linking ACS and HFpEF is that they commonly coexist and share multiple risk factors [42]. Each parameter included in the H2FPEF score is known to be associated with the pathophysiology of HFpEF. It is reasonable that the H2FPEF score exhibited predictive value for future ACS events in HFpEF patients.
In this study, by using the definition of obesity as BMI ≥ 25 kg/m2, we suggested that the best cutoff value was 6.5 for the modified H2FPEF score predicting both HF-related events and ACS attacks in Chinese HFpEF patients. A retrospective and single-center study in Japan, with the same definition of obesity, reported the cutoff H2FPEF score was 5.5 for identifying cardiovascular and HF-related events [17]. Nevertheless, Suzuki et al suggested the cutoff value was 7 points for the H2FPEF score to predict future HF-related events in stable outpatients with one or more cardiovascular risk factors. The symptoms of patients in the latter study were relatively mild compared to the HFpEF patients, which may result in a higher H2FPEF score in predicting the adverse outcomes. In the future, additional detailed, prospective, multicenter studies are warranted to verify this precise usefulness.
Each component of the H2FPEF score is simple, and its calculation is easy with a low cost in clinical practice, providing a wide range of potential applications. If this score further predicts subsequent cardiovascular events in HFpEF patients, like the application of CHA2DS2-VASc score in the AF, it would represent a valuable indicator for cardiologists in the clinical situation. Furthermore, from recent studies, HFpEF was classified into three leading phenotype groups, which are primarily based on aging, cardiometabolic stress, and chronic hypertension [15, 43, 44]. These subgroups exhibited significant differences in survival and potential treatment response [45]. Identification of HFpEF subgroups may help select specific interventions that, in turn, will improve the likelihood of a positive treatment response. Thus, further studies that help to promote the identification of HFpEF subgroups and targeted treatment are needed.
Study limitations
This study has several limitations. First, it was a single-center design with a relatively small Chinese population, which restricted the generalizability of the modified H2FPEF score. Second, the retrospective nature of these analyses creates a tremendous potential for bias. Prospective studies with a broader population are needed to validate the predictive utility of the modified H2FPEF score in real-time clinical trials. Third, although AF stood out as the most critical predictor of HFpEF in the H2FPEF score and concordantly received a 3-point score when positive, patients with high modified H2FPEF scores were all complicated with AF in our study, which may have caused a bias in the results. At last, we focused on different BMIs and did not consider other potentially relevant clinical variables or biomarkers that could enhance the prognostic accuracy. And it is unclear which factors contribute, and the extent of their contribution, to this classification and the prognosis of HFpEF. Thus, further pathophysiological and molecular physiological studies, including animal experiments, are warranted.
Conclusions
Using BMI ≥ 25 kg/m2 as the definition standard for obesity, the modified H2FPEF score can more effectively predict the occurrence of adverse cardiovascular events in Chinese HFpEF patients. The higher the score, the higher the risk of adverse cardiovascular events in HFpEF patients.
| Supplementary Material | ▴Top |
Figure 1. Kaplan-Meier analyses for primary (A) and secondary (B) endpoints according to H2FPEF scores by BMI ≥ 30 kg/m2. Receiver operating characteristic (ROC) curves for H2FPEF scores by BMI ≥ 30 kg/m2 to predict primary (C) and secondary (D) endpoints in HFpEF patients.
Figure 2. Kaplan-Meier analyses for cardiovascular death (A) and heart failure re-hospitalization (B) according to the modified H2FPEF scores (BMI ≥ 25 kg/m2). The 0-time point in the x-axis indicates discharge day of the qualifying heart failure hospitalization.
Table 1. Clinical characteristics according to H2FPEF score by BMI ≥ 30 kg/m2.
Table 2. Baseline characteristics of HFpEF patients according to the H2FPEF score by BMI ≥ 30 kg/m2.
Table 3. Cardiovascular events in the study groups according to the H2FPEF score by BMI ≥ 30 kg/m2.
Table 4. Cox proportional hazards analysis of cardiovascular events in HFpEF patient.
Table 5. Clinical characteristics according to the modified H2FPEF score.
Table 6. Comparison of the clinical characteristics between the groups with and without HF-related events in HFpEF patients.
Table 7. Comparison of the clinical characteristics between the groups with and without ACS in HFpEF patients.
Table 8. Sensitivity, specificity and likelihood ratio in the modified H2FPEF score for future HF-related events.
Table 9. Sensitivity, specificity and likelihood ratio in the modified H2FPEF score for future ACS.
Acknowledgments
None to declare.
Financial Disclosure
There was no specific funding source to be mentioned.
Conflict of Interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Informed Consent
All subjects provided written informed consent.
Author Contributions
YQJ and LG performed the statistical analysis and drafted the manuscript. KKW was responsible for software and data visualization. YL, MXW, and XYG collected the data. JZ, XCY and BX revised the manuscript. JCL designed and supervised the study. All authors provided critical comments on the manuscript. All authors read and approved the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ACS: acute coronary syndrome; AF: atrial fibrillation; AUC: area under the curve; BMI: body mass index; BNP: B-type natriuretic peptide; CI: confidence interval; eGFR: estimated glomerular filtration rate; HF: heart failure; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; HR: hazard ratio; LAD: left atrial diameter; LV: left ventricular; LVEF: left ventricular ejection fraction; LVMI: left ventricular mass index; MI: myocardial infarction; NSTEMI: non-ST elevation myocardial infarction; NT-proBNP: N-terminal pro B-type natriuretic peptide; PAP: pulmonary artery systolic pressure; RAD: right atrial diameter; ROC: receiver operating characteristic; SPSS: Statistical Package for Social Sciences; STEMI: ST elevation myocardial infarction; E/e’: ratio of early transmittal flow velocity to early diastolic mitral annular velocity; UA: uric acid
| References | ▴Top |
- Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2020;17(9):559-573.
doi pubmed - Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, Atherton JJ, Backs J, et al. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J. 2019;40(26):2155-2163.
doi pubmed pmc - Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017;70(20):2476-2486.
doi pubmed - Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020;22(8):1342-1356.
doi pubmed pmc - Vasan RS, Xanthakis V, Lyass A, Andersson C, Tsao C, Cheng S, Aragam J, et al. Epidemiology of left ventricular systolic dysfunction and heart failure in the framingham study: an echocardiographic study over 3 decades. JACC Cardiovasc Imaging. 2018;11(1):1-11.
doi pubmed pmc - Oktay AA, Shah SJ. Diagnosis and management of heart failure with preserved ejection fraction: 10 key lessons. Curr Cardiol Rev. 2015;11(1):42-52.
doi pubmed pmc - Gazewood JD, Turner PL. Heart failure with preserved ejection fraction: diagnosis and management. Am Fam Physician. 2017;96(9):582-588.
pubmed - Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251-259.
doi pubmed - Mishra S, Kass DA. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2021;18(6):400-423.
doi pubmed pmc - Henein MY, Vancheri S, Longo G, Vancheri F. The role of inflammation in cardiovascular disease. Int J Mol Sci. 2022;23(21).
doi pubmed pmc - Kumar V, Prabhu SD, Bansal SS. CD4(+) T-lymphocytes exhibit biphasic kinetics post-myocardial infarction. Front Cardiovasc Med. 2022;9:992653.
doi pubmed pmc - Vancheri F, Longo G, Henein MY. Left ventricular ejection fraction: clinical, pathophysiological, and technical limitations. Front Cardiovasc Med. 2024;11:1340708.
doi pubmed pmc - Kumar V, Rosenzweig R, Asalla S, Nehra S, Prabhu SD, Bansal SS. TNFR1 contributes to activation-induced cell death of pathological CD4(+) T lymphocytes during ischemic heart failure. JACC Basic Transl Sci. 2022;7(10):1038-1049.
doi pubmed pmc - Lewis GA, Schelbert EB, Williams SG, Cunnington C, Ahmed F, McDonagh TA, Miller CA. Biological phenotypes of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017;70(17):2186-2200.
doi pubmed - Roh J, Hill JA, Singh A, Valero-Munoz M, Sam F. Heart failure with preserved ejection fraction: heterogeneous syndrome, diverse preclinical models. Circ Res. 2022;130(12):1906-1925.
doi pubmed pmc - Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138(9):861-870.
doi pubmed pmc - Sueta D, Yamamoto E, Nishihara T, Tokitsu T, Fujisue K, Oike F, Takae M, et al. H2FPEF score as a prognostic value in HFpEF patients. Am J Hypertens. 2019;32(11):1082-1090.
doi pubmed - Suzuki S, Kaikita K, Yamamoto E, Sueta D, Yamamoto M, Ishii M, Ito M, et al. H(2) FPEF score for predicting future heart failure in stable outpatients with cardiovascular risk factors. ESC Heart Fail. 2020;7(1):65-74.
doi pubmed pmc - Churchill TW, Li SX, Curreri L, Zern EK, Lau ES, Liu EE, Farrell R, et al. Evaluation of 2 existing diagnostic scores for heart failure with preserved ejection fraction against a comprehensively phenotyped cohort. Circulation. 2021;143(3):289-291.
doi pubmed pmc - Sun Y, Wang N, Li X, Zhang Y, Yang J, Tse G, Liu Y. Predictive value of H(2) FPEF score in patients with heart failure with preserved ejection fraction. ESC Heart Fail. 2021;8(2):1244-1252.
doi pubmed pmc - W. H. O. E. Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157-163.
doi pubmed - Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982-992.
doi pubmed - Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277-314.
doi pubmed - Nagueh SF. Heart failure with preserved ejection fraction: insights into diagnosis and pathophysiology. Cardiovasc Res. 2021;117(4):999-1014.
doi pubmed - Toth PP, Gauthier D. Heart failure with preserved ejection fraction: disease burden for patients, caregivers, and the health-care system. Postgrad Med. 2021;133(2):140-145.
doi pubmed - Jih J, Mukherjea A, Vittinghoff E, Nguyen TT, Tsoh JY, Fukuoka Y, Bender MS, et al. Using appropriate body mass index cut points for overweight and obesity among Asian Americans. Prev Med. 2014;65:1-6.
doi pubmed pmc - Global BMIMC, Di Angelantonio E, Bhupathiraju Sh N, Wormser D, Gao P, Kaptoge S, Berrington de Gonzalez A, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776-786.
doi pubmed pmc - World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment. 2000.
- Tao Y, Wang W, Zhu J, You T, Li Y, Zhou X. H(2)FPEF score predicts 1-year rehospitalisation of patients with heart failure with preserved ejection fraction. Postgrad Med J. 2021;97(1145):164-167.
doi pubmed - Malik A, Masson R, Singh S, Wu WC, Packer M, Pitt B, Waagstein F, et al. Digoxin discontinuation and outcomes in patients with heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;74(5):617-627.
doi pubmed pmc - Llacer P, Nunez J, Bayes-Genis A, Conde Martel A, Cabanes Hernandez Y, Diez Manglano J, Alvarez Rocha P, et al. Digoxin and prognosis of heart failure in older patients with preserved ejection fraction: Importance of heart rate. Results from an observational and multicenter study. Eur J Intern Med. 2019;60:18-23.
doi pubmed - Eichhorn EJ, Lukas MA, Wu B, Shusterman N. Effect of concomitant digoxin and carvedilol therapy on mortality and morbidity in patients with chronic heart failure. Am J Cardiol. 2000;86(9):1032-1035.
doi pubmed - Lam PH, Packer M, Gill GS, Wu WC, Levy WC, Zile MR, Brar V, et al. Digoxin initiation and outcomes in patients with heart failure with preserved ejection fraction. Am J Med. 2020;133(10):1187-1194.
doi pubmed pmc - Singh S, Moore H, Karasik PE, Lam PH, Wopperer S, Arundel C, Tummala L, et al. Digoxin Initiation and Outcomes in Patients with Heart Failure (HFrEF and HFpEF) and Atrial Fibrillation. Am J Med. 2020;133(12):1460-1470.
doi pubmed - Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, Josephson RA, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509-1513.
doi pubmed - Murphy SA. When 'digoxin use' is not the same as 'digoxin use': lessons from the AFFIRM trial. Eur Heart J. 2013;34(20):1465-1467.
doi pubmed - Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175(6):996-1004.
doi pubmed pmc - Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124(11):1598-1617.
doi pubmed pmc - Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63(25 Pt A):2817-2827.
doi pubmed - Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40(40):3297-3317.
doi pubmed - Cordero A, Rodriguez-Manero M, Bertomeu-Gonzalez V, Garcia-Acuna JM, Baluja A, Agra-Bermejo R, Alvarez-Alvarez B, et al. New-onset heart failure after acute coronary syndrome in patients without heart failure or left ventricular dysfunction. Rev Esp Cardiol (Engl Ed). 2021;74(6):494-501.
doi pubmed - Cunningham JW, Vaduganathan M, Claggett BL, John JE, Desai AS, Lewis EF, Zile MR, et al. Myocardial infarction in heart failure with preserved ejection fraction: pooled analysis of 3 clinical trials. JACC Heart Fail. 2020;8(8):618-626.
doi pubmed - Cohen JB, Schrauben SJ, Zhao L, Basso MD, Cvijic ME, Li Z, Yarde M, et al. Clinical phenogroups in heart failure with preserved ejection fraction: detailed phenotypes, prognosis, and response to spironolactone. JACC Heart Fail. 2020;8(3):172-184.
doi pubmed pmc - Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134(1):73-90.
doi pubmed pmc - Kao DP, Lewsey JD, Anand IS, Massie BM, Zile MR, Carson PE, McKelvie RS, et al. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail. 2015;17(9):925-935.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


